Microprotein Adipogenin Emerges as a Key Regulator of How Fat Cells Store Lipids
A new study has uncovered a surprisingly powerful role for a tiny microprotein called adipogenin in controlling how fat cells store lipids. Even though this microprotein is made of only 80 amino acids, researchers found that it has an outsized influence on the formation and growth of lipid droplets, which are the primary storage structures for fats inside adipocytes. The discovery sheds new light on how the body manages energy storage and could pave the way for new treatments targeting conditions such as obesity, diabetes, lipodystrophy, and fatty liver disease.
The work was co-led by scientists at UT Southwestern Medical Center in collaboration with the University of Helsinki, and the findings were published in Science. The study focuses on the relationship between adipogenin and a well-known protein called seipin, long recognized as essential for healthy lipid droplet formation. But until now, seipin’s exact mechanism of action has been unclear. This research offers a clear answer: adipogenin binds directly to a dodecameric seipin complex, stabilizing its structure and enabling fat cells to form larger and healthier lipid droplets.
How Fat Storage Works and Why It Matters
Whenever we eat, the body breaks down dietary fats into lipids. Some of these lipids are used immediately for energy, but the rest must be stored. The safest and most efficient place for storage is inside fat cells (adipocytes). These cells package lipids inside spherical structures known as lipid droplets—similar to how oil clusters into droplets when mixed with water.
If lipids accumulate outside fat cells, such as in the liver, muscles, or pancreas, they can cause lipotoxicity, a damaging process that leads to inflammation, cell death, and various metabolic disorders. That’s why proper lipid droplet formation is essential for metabolic health. The new study reveals that adipogenin is a central player in making this happen.
The Role of Seipin and Why Adipogenin Matters
Previous work established that seipin is a crucial scaffolding protein that guides the early steps of lipid droplet formation. Organisms including plants, fungi, and mammals all rely on seipin, showing how deeply conserved its function is.
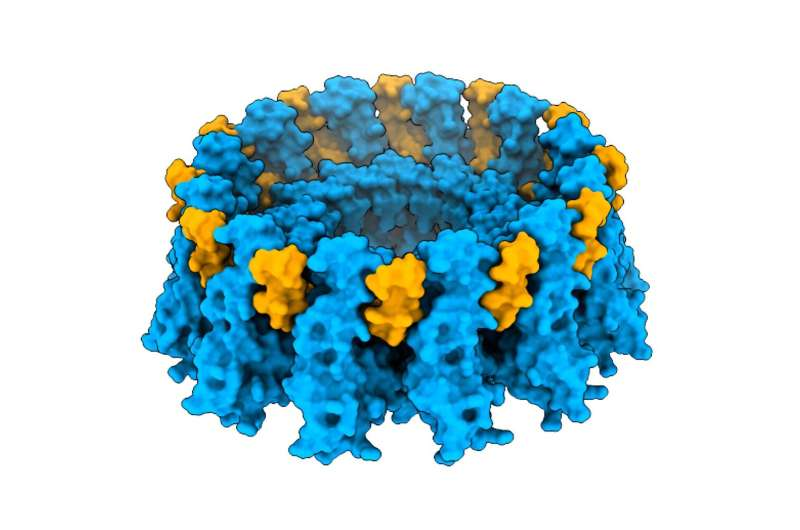
But seipin does not work alone. The new research found that adipogenin binds directly to seipin, which usually forms large circular structures made of multiple identical units. In particular, adipogenin prefers to interact with a 12-unit seipin complex, known as a dodecamer. Using cryo-electron microscopy, the scientists were able to visualize the adipogenin-seipin interaction at atomic resolution and observed that adipogenin reinforces the structure of the complex, making it more rigid and stable.
This stabilizing effect allows the seipin complex to better support the formation and growth of lipid droplets. Without adipogenin, the structure is weaker and less efficient at storing lipids.
What Experiments in Mice Revealed
To understand adipogenin’s function in a living organism, the research team conducted experiments on mice. These experiments included mice engineered to overproduce adipogenin and others engineered to lack adipogenin entirely.
Key findings include:
- Mice with extra adipogenin developed larger lipid droplets in their fat cells.
- These mice stored significantly more fat overall compared to normal mice.
- Mice without adipogenin formed much smaller lipid droplets and carried less fat mass.
- The absence of adipogenin disrupted how efficiently fat cells expanded and managed incoming lipids.
This clear contrast showed that adipogenin is not just a minor participant—it is a central regulator of lipid droplet expansion and energy storage.
Why This Discovery Is Important for Human Health
Many metabolic diseases stem from improper fat storage. For example:
- In obesity, fat cells may expand beyond their healthy capacity, causing inflammation and metabolic stress.
- In diabetes, faulty lipid handling can interfere with insulin signaling.
- In lipodystrophy, the body cannot form or maintain healthy fat tissue, forcing lipids into organs where they don’t belong.
- In fatty liver disease, excessive lipids accumulate in the liver due to poor regulation elsewhere.
Because adipogenin offers a direct way to influence how fat cells store lipids, it presents a new potential therapeutic target. Tweaking adipogenin activity might allow scientists to:
- Reduce harmful fat buildup by limiting lipid droplet expansion where necessary.
- Boost healthy fat storage in conditions where adipocytes cannot store lipids properly.
- Improve overall metabolic stability by preventing lipids from accumulating in vulnerable organs.
The researchers emphasized that adipogenin could become a “druggable lever” to fine-tune seipin’s function—something that has not been possible before.
Expanding the Understanding of Microproteins
One of the broader implications of this study is the recognition that microproteins—proteins made of fewer than 100 amino acids—play crucial biological roles that have long gone unnoticed. Many microproteins were only recently discovered, and scientists are still uncovering their functions.
Adipogenin’s importance highlights how these tiny molecules can act as major regulators of cellular machinery. As more microproteins are studied, it’s likely that even more unexpected mechanisms will be uncovered, particularly in areas related to metabolism, cell stress responses, and organ development.
Additional Background on Lipid Droplets and Seipin
To make the study’s findings clearer, it helps to understand what lipid droplets are made of and how they form.
Lipid droplets consist of:
- A core of neutral lipids, mainly triglycerides and cholesterol esters.
- A phospholipid monolayer that wraps around this oily core.
- Protein regulators, like seipin and adipogenin, that control droplet size and function.
Seipin’s known functions include:
- Defining the site where lipid droplets begin to form.
- Preventing the formation of too many small, dysfunctional droplets.
- Ensuring droplets grow uniformly and remain stable.
With adipogenin now identified as a partner that supports and stabilizes seipin’s structure, scientists have a clearer blueprint of how lipid droplets grow from tiny seeds into mature storage units.
What Happens Next in This Line of Research
Future research will likely explore:
- Whether manipulating adipogenin or seipin can treat specific metabolic disorders.
- How adipogenin levels change in obesity, fasting, or high-fat diets.
- Whether different tissues express unique variants of adipogenin.
- How adipogenin interacts with other proteins involved in lipid metabolism.
This discovery opens an entirely new avenue for understanding how the body manages fat storage—and how those processes can be corrected when they go wrong.
Research Paper Link
Adipogenin promotes the development of lipid droplets by binding a dodecameric seipin complex
https://www.science.org/doi/10.1126/science.adr9755





