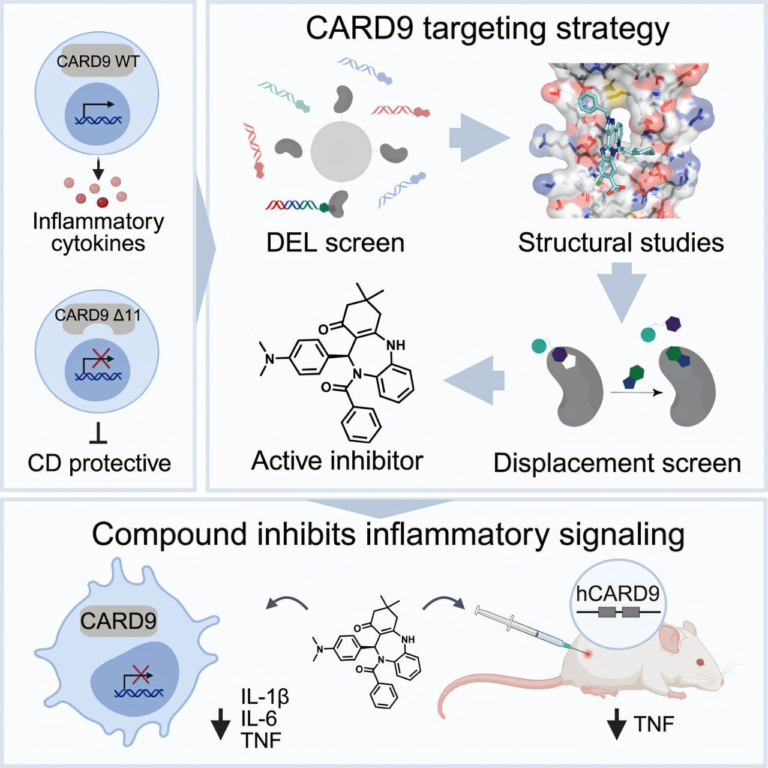
New Genetic Testing Breakthrough Offers a Complete View of Arrhythmia and Sudden Cardiac Death Risk
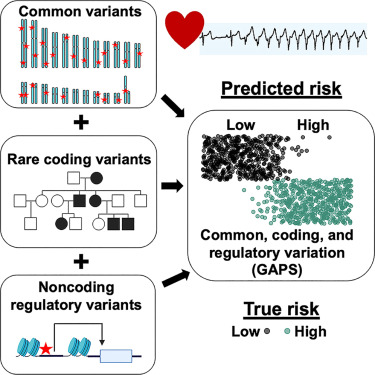
A new study from Northwestern Medicine has introduced a powerful and refreshingly comprehensive approach to predicting a person’s risk of arrhythmia and sudden cardiac death, combining three major types of genetic testing into one unified system. Instead of looking at just rare mutations or just common variants—or scanning the whole genome without fully integrating context—the researchers blended all three to create what they call a 360-degree genetic risk score. This study, which included 1,119 participants and was published on November 11, 2025, in Cell Reports Medicine, could reshape how we think about personalized heart care and even how genetic testing is used across many fields of medicine.
A Clear Look at the Three Genetic Layers Used in the Study
Doctors and researchers typically rely on three kinds of genetic testing, each valuable but often used separately:
- Monogenic testing
This detects rare mutations in a single gene, similar to finding a typo in an important sentence. For years, this type of testing has been commonly used for inherited heart conditions. However, many patients with arrhythmias do not carry these rare, clearly damaging mutations. - Polygenic testing
This examines common gene variants across the genome. Each variant contributes a tiny bit of risk, and when added together, they paint a broader risk picture—something like analyzing the overall tone of a long book. - Genome sequencing
This reads the entire genetic code, providing the raw ingredients for deeper analysis, including the parts of DNA that don’t code for proteins but still play major regulatory roles.
What makes the new study exciting is how the researchers combined all three. They didn’t treat them as isolated silos; instead, they layered them together to quantify risk more precisely than any method used alone.
How Northwestern Scientists Built the New Risk Score
The research team, led by Dr. Elizabeth McNally, brought together a group of 523 participants who had confirmed arrhythmias. Some also had heart failure, and the team rigorously checked each medical record, device reading, and clinical note to ensure that every participant met the criteria. This careful review process was described as painstaking, underscoring how thoroughly the team validated the data.
These participants underwent full genome sequencing, which allowed the scientists to examine:
- Rare coding mutations
- Common genetic variants used for polygenic scores
- Non-coding mutations that could affect gene regulation
- Gene panels focused on cardiomyopathy and arrhythmia pathways
To compare their findings, researchers evaluated these results against 596 control participants from the NUgene biobank. These controls were aged 40 and older, with no known history of cardiac disease.
By blending all these pieces, the researchers created what is essentially a combined genomic arrhythmia propensity score. This score highlights individuals who may be at significantly elevated risk long before symptoms show up on traditional tests like ECGs or echocardiograms.
Why This Matters for Physicians and Patients
Most cardiologists rely on symptoms, family history, and standard diagnostics to assess heart risk. Genetic testing is used far less often—even when it could drastically improve risk detection.
According to the study team, only 1% to 5% of people who should get genetic testing for heart conditions actually receive it. Even in cancer care—where genetic links are well-established—only 10% to 20% undergo testing.
This means that a large percentage of high-risk individuals remain unidentified.
A more complete risk score like the one developed in this study makes it possible for physicians to:
- Predict arrhythmia risk before symptoms appear
- Identify patients who may benefit from early interventions such as defibrillators
- Tailor treatment to match a patient’s full genetic profile, not just a slice of it
The researchers believe their approach could transform not only cardiology but also how genetic data is interpreted across many medical specialties.
Why Combining All Three Genetic Approaches Is Such a Big Deal
When someone undergoes only monogenic testing, physicians may only find something if the person has one of the rare, powerful mutations. But most people don’t. And yet many still experience arrhythmia or even sudden cardiac death.
Similarly, polygenic scores alone don’t capture rare damaging mutations or subtle non-coding influences.
Genome sequencing provides the entire blueprint, but without a structured way to interpret which parts matter, it becomes noisy and overwhelming.
Putting the three together creates a much richer picture. In this study, combining rare variants, common variants, and non-coding variants delivered a significantly stronger odds ratio in correctly identifying who is actually at highest risk. Hidden patterns in DNA that might be missed by one testing approach suddenly become visible when all layers are analyzed together.
What This Means Beyond Arrhythmia
While the focus here is on arrhythmia and sudden cardiac death, the researchers make it clear that this methodology is not limited to heart disease. Because many diseases—like cancer, Parkinson’s disease, and autism—involve multiple genetic influences, a similar integrated approach could reveal patterns that single-method testing would overlook.
Dr. McNally described the work as a roadmap that researchers and genetic testing companies can apply to virtually any condition influenced by a mix of genetic factors.
Understanding Arrhythmias and Why Predicting Them Is Hard
Arrhythmias are irregular heartbeats caused by problems with the heart’s electrical system. They range from harmless skipped beats to severe conditions such as atrial fibrillation (AFib) and ventricular arrhythmias that can lead to sudden cardiac arrest.
A major challenge is that arrhythmias can strike:
- Without warning
- Without prior symptoms
- In people who appear otherwise healthy
In a large number of cases, traditional tests fail to reveal underlying risk. That’s why a tool like the new combined genetic score could be a critical addition to preventive cardiology.
Extra Insight: Why Non-Coding DNA Matters More Than People Think
Because only about 1–2% of human DNA directly codes for proteins, the rest—once called “junk DNA”—was long overlooked. But research over the past decade has shown that non-coding regions contain:
- Regulatory switches
- Enhancers and silencers
- Sequences that control when and where genes are active
A single non-coding variant can disrupt gene expression enough to increase disease risk. But that subtle influence is almost impossible to detect using routine genetic testing.
The new study’s inclusion of non-coding genome information helps fill this gap and is part of what makes the approach so effective.
The Team Behind the Study
The research included contributions from:
- Tanner Monroe
- Megan Puckelwartz
- Lorenzo Pesce
- Dr. Alfred George
- Dr. Gregory Webster
Their combined expertise spans cardiology, genetic testing, molecular genetics, and statistical genomics.
What This Breakthrough Could Mean for the Future of Heart Health
If adopted widely, this kind of integrated genetic testing could influence how healthcare systems screen for heart disease risk. In the future, instead of waiting for symptoms—or relying solely on family history—clinicians could use combined genetic scores as a routine early detection tool.
This could lead to:
- Earlier intervention
- More personalized treatment
- Reduced sudden cardiac death events
- Better long-term heart health outcomes
The study provides a major step toward that future.
Research Reference:
A Combined Genomic Arrhythmia Propensity Score Delineates Cumulative Risk
https://doi.org/10.1016/j.xcrm.2025.102455