New LED-Based Therapy Destroys 92% of Skin Cancer Cells Without Damaging Healthy Tissue
A team of scientists from The University of Texas at Austin and the University of Porto in Portugal has developed a powerful new way to fight cancer — using LED light and microscopic flakes of tin to kill tumor cells while leaving healthy tissue untouched. The results are impressive: in lab tests, the treatment eliminated up to 92% of skin cancer cells and 50% of colorectal cancer cells, with no harm to healthy skin cells.
This new method, built on the principles of near-infrared photothermal therapy, could become a safer, cheaper, and more accessible alternative to traditional cancer treatments like chemotherapy or laser-based therapy. The research, published in ACS Nano, details how these tin-based “SnOx nanoflakes” are activated by LED light to heat and neutralize cancer cells — all without the painful side effects that come with many current therapies.
How the Therapy Works
At the heart of this breakthrough are SnOx nanoflakes, tiny two-dimensional particles made from tin compounds. To create them, the research team started with tin disulfide (SnS₂) powders, which are normally not very reactive under near-infrared (NIR) light. They then used an electrochemical oxidation process to convert these powders into a more reactive form — a combination of tin oxide (SnO and SnO₂) layers known collectively as SnOx.
These nanoflakes are incredibly thin (less than 20 nanometers thick) and small (under 400 nanometers wide), yet they have extraordinary properties. When exposed to NIR LED light, they efficiently absorb the light and convert it into localized heat. Cancer cells, being more vulnerable to heat stress than healthy cells, are selectively destroyed.
In experiments, a simple dispersion of SnOx nanoflakes in water — at just 3 mg/mL concentration — was able to heat up by about 19°C within 30 minutes of NIR LED exposure. Even at lower concentrations, the photothermal conversion efficiency reached an impressive 93%, which means that nearly all of the absorbed light was successfully turned into heat.
What makes this approach particularly interesting is that the nanoflakes remained stable after multiple cycles of light exposure. This means the material could be reused without losing effectiveness, a key factor for scalability and long-term clinical applications.
Results from the Study
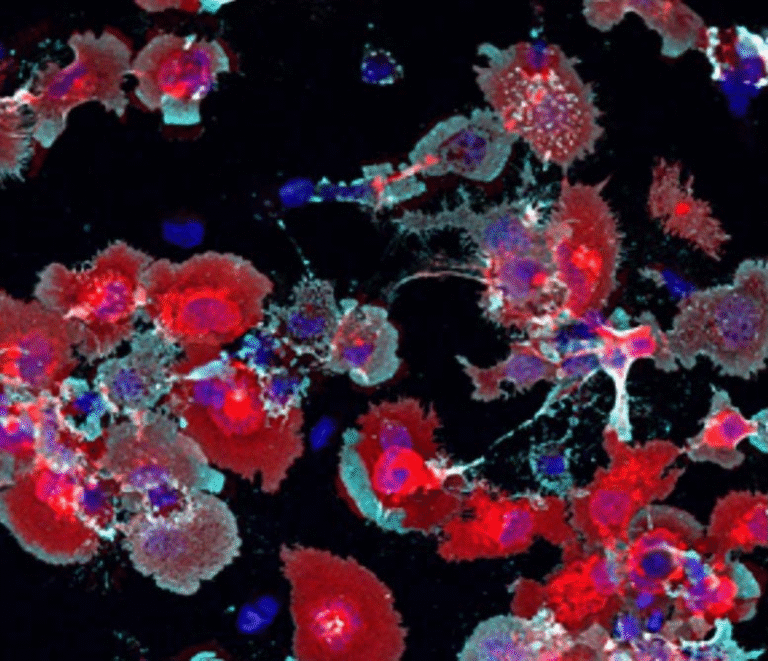
The researchers tested this LED-based system on two types of cancer cells — skin carcinoma (A431) and colorectal cancer (SW837) — and compared the results with healthy human skin fibroblast cells (HFF-1).
After just 30 minutes of LED exposure (using light intensity around 115 mW/cm² and a wavelength near 810 nm), the system destroyed 92% of skin cancer cells and 50% of colorectal cancer cells. Importantly, it caused no observable damage to healthy skin cells.
This selectivity is one of the most promising aspects of the therapy. It means that the treatment targets only the harmful cells while sparing normal tissue — something conventional methods like chemotherapy struggle to achieve.
The study confirms that the combination of LED light and SnOx nanoflakes can precisely heat and kill tumor cells without collateral damage. This approach opens a path toward non-invasive or minimally invasive cancer treatment with fewer side effects, less pain, and lower costs.
Why LEDs Instead of Lasers?
Traditional photothermal therapy (PTT) typically relies on lasers to activate the nanomaterials that heat and destroy cancer cells. While effective, laser systems come with significant drawbacks:
- High cost – Lasers and their safety equipment are expensive and require specialized facilities.
- Limited accessibility – Many hospitals, especially in developing regions, lack the infrastructure for advanced laser setups.
- Tissue safety issues – Lasers can cause unintended burns or damage to nearby healthy tissue if not precisely controlled.
The new study replaces lasers with LEDs, which are cheaper, more compact, and safer. LEDs can be built into portable, easy-to-use systems, allowing for treatments that don’t require complex medical environments.
This innovation could make light-based cancer therapy available to far more patients around the world. The research team even envisions home-use devices that could treat residual cancer cells after surgery — particularly for skin cancers.
For example, a small, wearable LED device could one day be placed over a patient’s skin after tumor removal. The device would deliver safe doses of NIR light to heat up the nanoflakes that remain in the tissue, killing any leftover cancer cells and reducing the risk of recurrence.
The Collaboration Behind the Discovery
This project is part of the UT Austin Portugal Program, a long-term collaboration that connects researchers from the University of Texas at Austin with teams in Portugal to work on cutting-edge technologies.
Professor Jean Anne Incorvia from UT Austin’s Cockrell School of Engineering and Professor Artur Pinto from the University of Porto’s Faculty of Engineering led the joint effort. Since their partnership began in 2021, the two groups have exchanged researchers, shared laboratory methods, and developed several innovations using two-dimensional materials — the same family of materials that includes graphene.
This specific work received new funding in 2025 to expand its scope, including efforts to design a biocompatible implant for breast cancer treatment using similar principles.
Why Photothermal Therapy Matters
Cancer remains the second leading cause of death globally, and current treatments often come with devastating side effects. Chemotherapy, for example, affects both cancerous and healthy cells, leading to fatigue, hair loss, nausea, and immune suppression. Radiation therapy and surgery can also cause pain, scarring, and complications.
Photothermal therapy (PTT) represents a promising alternative. It works by using light to heat up nanoparticles that are selectively delivered to cancer cells. When these particles absorb light, they convert it to heat, raising the temperature of cancer cells until they die.
The advantages are clear:
- Non-invasive or minimally invasive procedure
- Precise targeting of tumor cells
- Fewer side effects compared to drugs or radiation
- Fast treatment times (often within minutes)
The challenge, until now, has been making this process safe, affordable, and widely accessible. The new LED-based system addresses those problems head-on.
Potential Applications and Next Steps
While these results are highly encouraging, the therapy is still in the early stages of development. The current work has only been performed in vitro, meaning in cell cultures, not yet in live animals or humans.
The next steps will involve:
- Testing in animal models to see how the treatment behaves in a full biological system.
- Ensuring targeted delivery, so the SnOx nanoflakes accumulate only in tumor regions.
- Studying long-term safety, including how the body processes or removes any leftover tin oxide materials.
- Designing a functional device, possibly a portable or wearable LED system for clinical and at-home use.
Researchers also plan to test different types of nanomaterials that may enhance heat generation or improve targeting precision. By experimenting with new catalysts and optimizing the light–heat conversion, they hope to make this therapy even more effective.
How It Differs from Traditional Treatments
Unlike chemotherapy or radiation therapy, this LED-based photothermal therapy does not rely on systemic drugs or ionizing radiation. Instead, it depends on localized heat and targeted energy delivery.
In chemotherapy, toxic drugs circulate throughout the body, damaging both cancerous and healthy cells. Radiation, meanwhile, can affect nearby tissues and requires precise imaging and positioning to avoid harm.
Here, the energy source is external, and the material does the work at the tumor site. The process could complement existing treatments — for instance, used after tumor surgery to eliminate remaining cancer cells, or in combination with drugs to improve outcomes.
Moreover, since LEDs are relatively low-intensity and operate at near-infrared wavelengths, they can safely penetrate tissue without the same risks that come with high-energy lasers.
Challenges Ahead
Of course, there are hurdles to overcome before this can reach hospitals or homes. Among them:
- Penetration depth: NIR light can only go a few centimeters into tissue, so deeper tumors will require special delivery methods (like fiber optics).
- Regulatory approval: Medical devices using nanomaterials must undergo extensive testing for toxicity, durability, and effectiveness.
- Consistency of results: Scaling up from lab conditions to real-world applications requires reproducibility and clinical validation.
- Long-term safety: Researchers must ensure that tin oxide nanoflakes don’t accumulate in the body or trigger immune reactions.
Despite these challenges, the potential impact is huge. The combination of low-cost LEDs, efficient photothermal nanomaterials, and non-invasive delivery could open the door to safer, more affordable cancer treatments around the world.
A Step Toward Accessible Cancer Therapy
What makes this discovery stand out is its accessibility. Cancer therapies are often complex, costly, and limited to high-tech hospitals. This research suggests that, one day, treating certain types of cancer could be as simple as applying a portable LED patch — a revolutionary idea in the fight against one of humanity’s deadliest diseases.
By merging nanotechnology, engineering, and medicine, the team from UT Austin and the University of Porto has taken a bold step toward that future.
The collaboration not only highlights what’s possible when global research teams unite, but also signals a shift toward democratizing advanced cancer care — bringing effective, side-effect-free therapies to patients everywhere.
Research Reference:
“SnOx Nanoflakes as Enhanced Near-Infrared Photothermal Therapy Agents Synthesized from Electrochemically Oxidized SnS₂ Powders” – ACS Nano, 2025