New Neuroscience Research Shows How Neurons Produce GABA Far From Synapses Without Disrupting Brain Communication
Recent neuroscience research from Colorado State University (CSU) is offering a much clearer picture of how neurons manage chemical balance in the brain, particularly when it comes to inhibitory signaling. The findings shed light on how brain cells remain stable and communicative even when key neurotransmitters are produced far from where they are ultimately used. This work could prove especially important for understanding and treating conditions such as epilepsy, autism, and schizophrenia.
At the center of this research is GABA (gamma-aminobutyric acid), one of the brain’s most important neurotransmitters. GABA plays a calming role in neural circuits, preventing excessive firing of neurons and maintaining balance within the brain’s complex communication networks. When this balance is disrupted, neurological and psychiatric disorders can emerge.
The study was led by Associate Professor Soham Chanda from CSU’s Department of Biochemistry and Molecular Biology, in collaboration with Matthew Xu-Friedman from the University at Buffalo and Thomas Bartol from the Salk Institute. The research was published in The Journal of Neuroscience and also included contributions from former CSU undergraduate students Orion Benner and Charles Karr.
Understanding How Neurons Communicate
Neurons are highly specialized cells responsible for sending and receiving information throughout the brain and nervous system. They rely on both electrical impulses and chemical signals to regulate everything from muscle movement to mood, memory, and perception.
Communication between neurons happens at junctions called synapses. At these sites, neurotransmitters are released from one neuron and received by another, allowing signals to pass across neural networks. Traditionally, scientists believed that neurotransmitters like GABA needed to be produced very close to these synapses to function effectively.
This new research challenges that assumption.
The Role of GABA in Brain Stability
GABA is the brain’s primary inhibitory neurotransmitter, meaning it reduces neuronal excitability. In simple terms, GABA helps prevent the brain from becoming overstimulated. Without enough GABA activity, neurons can fire uncontrollably, a hallmark of disorders such as epilepsy.
GABA is synthesized from glutamate by enzymes known as glutamate decarboxylase (GAD). There are two major forms of this enzyme: GAD65 and GAD67. These enzymes have long been known to play a role in GABA production, but their exact locations within neurons — and how much that location matters — has remained unclear.
What the Study Set Out to Discover
The CSU-led research focused on understanding whether the location of GABA-producing enzymes inside neurons affects the ability of neurons to communicate properly.
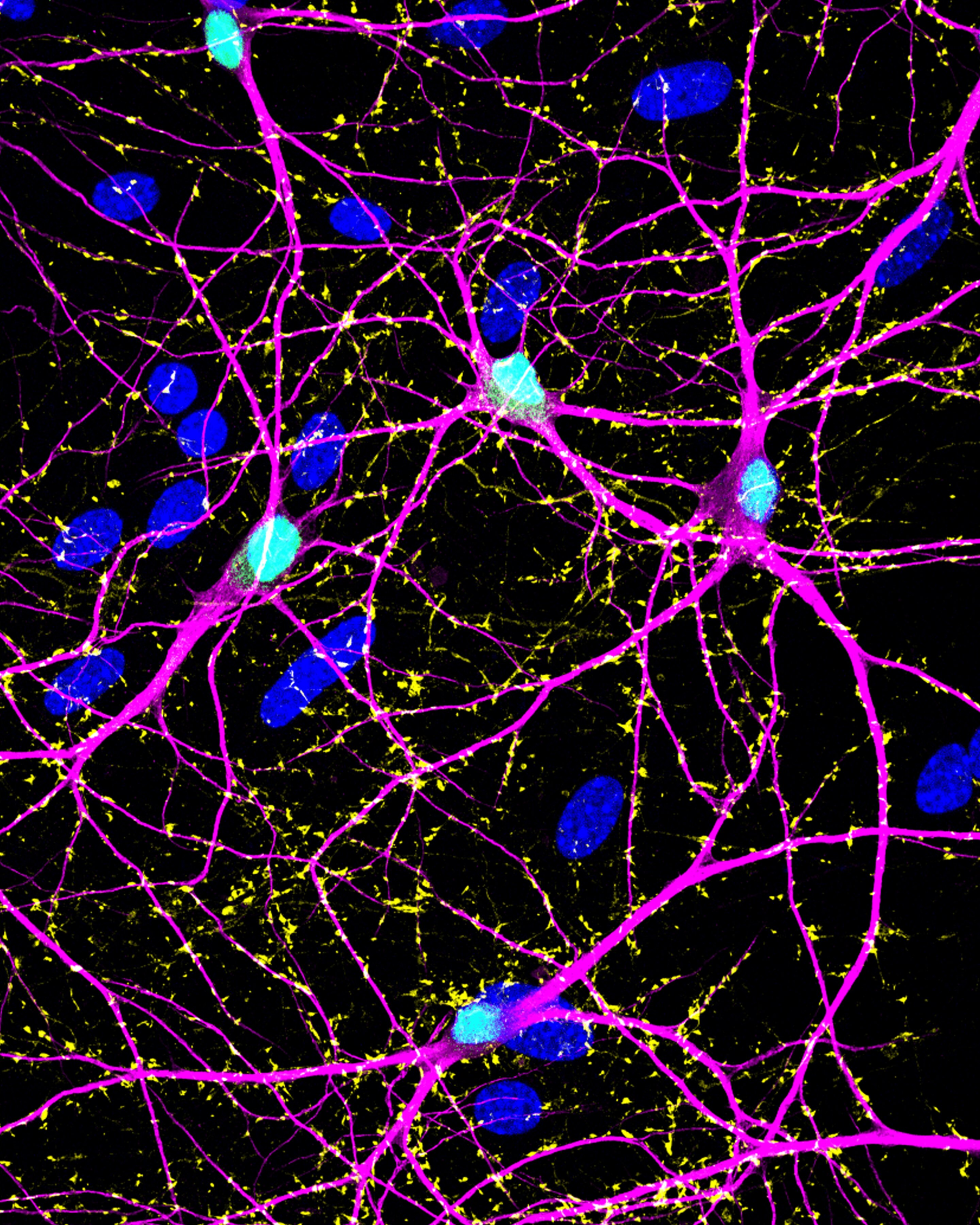
To investigate this, researchers engineered human neurons to express recombinant enzymes capable of producing GABA specifically in the nucleus of the cell. These neurons were grown alongside astrocytes, another important type of brain cell that supports neuronal health and signaling.
The researchers then examined whether GABA produced far from synapses — even deep within the nucleus — could still support normal neurotransmission at synapses located at distant parts of the neuron.
Key Findings From the Research
One of the most important discoveries was that neurons remained fully capable of basic GABAergic neurotransmission, even when GABA synthesis occurred far from the synapse.
The study showed that:
- Both GAD65 and GAD67 were able to generate sufficient levels of GABA inside neurons.
- GABA produced away from synaptic sites was still able to reach synapses and support inhibitory signaling.
- Differences in how GAD65 and GAD67 were distributed within neurons did not significantly alter baseline inhibitory communication.
- The presynaptic trafficking of these enzymes, once thought to be essential, was found to be dispensable for maintaining basic GABA signaling.
In simpler terms, neurons appear to be far more flexible than previously believed. They can produce GABA in one part of the cell and still deliver it effectively to synapses where it is needed.
Why This Changes How Scientists Think About Synapses
For years, neuroscience research emphasized precise molecular positioning at synapses as a requirement for proper neurotransmission. This study suggests that, at least for basal GABAergic signaling, neurons do not need such strict spatial organization.
This insight helps explain how neurons maintain stable communication even under conditions where internal cellular organization is altered. It also highlights a previously underappreciated robustness in brain circuitry, which may protect neural networks from minor disruptions.
Implications for Neurological and Psychiatric Disorders
Disruptions in GABA signaling are linked to a wide range of conditions, including epilepsy, autism spectrum disorders, schizophrenia, and other psychiatric illnesses. In many of these disorders, neurotransmitter production, release, or balance is impaired.
By clarifying how neurons synthesize and distribute GABA, this research provides a deeper understanding of what can go wrong in diseased brains. More importantly, it offers clues about potential therapeutic strategies that focus on restoring proper neurotransmitter balance rather than targeting only synaptic structures.
Understanding that GABA synthesis does not need to occur directly at synapses opens the door to new ways of thinking about drug targets and treatment approaches.
How This Study Fits Into Ongoing Research
Chanda’s lab has been exploring the molecular and cellular mechanisms of synaptic function since 2019. This paper builds directly on earlier work published in Nature Communications in 2022 and Proceedings of the National Academy of Sciences (PNAS) in 2024.
Together, these studies aim to define the fundamental principles governing human synapse properties, with a particular focus on how neurotransmitter synthesis, localization, and release shape information processing in the brain.
A Closer Look at Astrocytes and Neuronal Support
An important aspect of this study was the use of astrocytes in the experimental system. Astrocytes are non-neuronal brain cells that play a critical role in regulating neurotransmitter levels, maintaining metabolic support, and stabilizing synaptic environments.
By co-culturing neurons with astrocytes, the researchers created a more realistic model of the human brain, strengthening the relevance of their findings to real-world neurological conditions.
Why This Research Matters Going Forward
This study contributes valuable knowledge about how healthy brain circuits maintain balance, even when molecular processes occur far from their traditional locations. It reinforces the idea that brain cells are highly adaptable systems capable of maintaining function under varying conditions.
For researchers and clinicians alike, these insights help narrow the gap between basic neuroscience research and clinical applications, potentially guiding future therapies aimed at restoring normal inhibitory signaling in the brain.
As neuroscience continues to uncover the fine details of synaptic regulation, studies like this remind us that the brain often operates with more flexibility and resilience than previously assumed.
Research Paper Reference:
Presynaptic trafficking of Glutamate Decarboxylase isoforms is dispensable for basal GABAergic neurotransmission — The Journal of Neuroscience (2025)
https://doi.org/10.1523/JNEUROSCI.1043-25.2025