New Research Shows How the Drug Fexinidazole Kills Sleeping Sickness Parasites by Damaging Their DNA
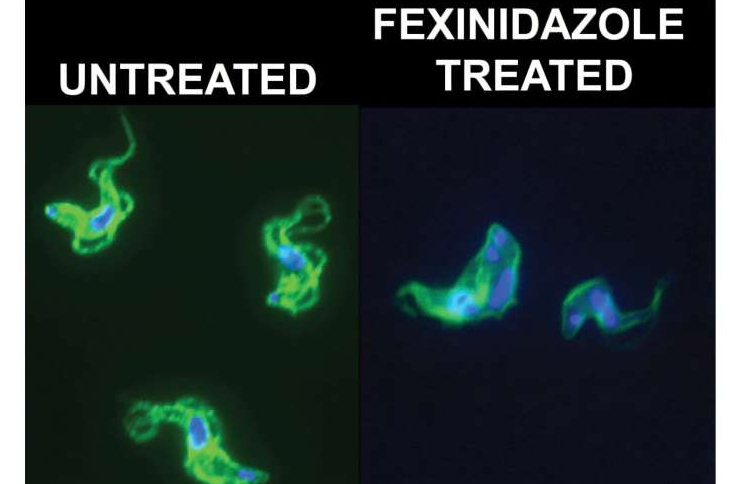
A new scientific study has finally clarified how the drug fexinidazole, the first fully oral treatment for Human African Trypanosomiasis (HAT)—better known as sleeping sickness—actually kills the parasites responsible for this deadly infection. Until now, researchers knew the drug worked but did not understand the precise biological mechanism behind its effectiveness. The new analysis, led by scientists at Stony Brook University and recently published in PLOS Neglected Tropical Diseases, uncovers direct evidence that fexinidazole kills Trypanosoma brucei parasites by damaging their DNA and shutting down DNA synthesis, ultimately causing the cells to fail and die.
This discovery is especially important because HAT is one of the most dangerous parasitic diseases in sub-Saharan Africa. Without treatment, the infection is almost always fatal. Historically, available treatments have been difficult to administer, had harsh side effects, or produced only mixed results. Fexinidazole became a major breakthrough when it was introduced as the first oral monotherapy for sleeping sickness, simplifying treatment and improving accessibility. Now, researchers have provided the first full picture of how this drug destroys parasites at the cellular level, giving scientists new tools to design even stronger therapies in the future.
How Fexinidazole Actually Kills the Parasite
The Stony Brook University team used advanced cell biology techniques to follow what happens inside Trypanosoma brucei cells after exposure to fexinidazole. Their findings showed that the drug triggers accumulation of DNA damage, leading to parasites with unusually large or misshapen nuclei—clear signs of a failing DNA replication process. These structural abnormalities indicate the parasites can no longer maintain or copy their genetic material. The study further confirmed that fexinidazole significantly slows and disrupts DNA synthesis, which directly contributes to cell death.
For decades, many anti-parasitic drugs classified as nitroaromatic compounds (including those used to treat related diseases like Chagas disease) have been thought to work primarily by generating reactive oxygen species (ROS) that stress and damage parasite cells. The new study clarifies that while ROS may still play a role, fexinidazole’s defining action is its ability to interfere specifically with DNA integrity. The researchers also compared fexinidazole with two earlier nitroaromatic drugs—nifurtimox and benznidazole—and found that fexinidazole produces unique patterns of DNA damage and cell-cycle disruption.
Understanding this mechanism is crucial, not just for improving sleeping sickness treatments but also for predicting and managing drug resistance. If scientists know exactly which pathways fexinidazole affects, they can study how parasites might evolve to avoid these effects—and design new drugs that block those escape routes.
Why This Matters for Global Health
More than 1 billion infections caused by trypanosomatid parasites occur worldwide each year, making this family of diseases a major global health concern. Sleeping sickness is caused by African trypanosomes transmitted through tsetse flies. American trypanosomes, on the other hand, cause Chagas disease, which leads to chronic heart complications in up to 30% of infected individuals.
Previously, Chagas disease was mostly restricted to Central and South America. However, as of September 2025, the CDC has reported that the infection has become endemic in the southern United States, a development linked to climate change and expanding habitats for vector insects. Because only two drugs—nifurtimox and benznidazole—are currently available for Chagas disease, and both have toxicity concerns and variable outcomes, the discovery that fexinidazole can kill parasites through DNA damage opens up important new possibilities. The study’s authors suggest that fexinidazole or similar DNA-targeting drugs may become valuable tools as Chagas disease spreads into new regions.
Importantly, the biological features shared by trypanosomatid parasites—including their genomic structures, metabolic pathways, and drug-activation mechanisms—mean that insights gained from studying HAT parasites can help create new treatments for related infections. The Stony Brook research group is already using genetics-based strategies to identify genes linked to drug resistance, mitochondrial stress, and other cellular changes that influence how parasites respond to treatment. These efforts could lead to the discovery of new therapeutic targets in the future.
The Growing Challenge of Trypanosomatid Infections
Sleeping sickness and Chagas disease are part of a broader group of difficult-to-treat parasitic diseases. African trypanosomes cause neurological deterioration, confusion, sleep disruptions, and eventually coma if untreated. Chagas disease progresses slowly but can cause heart failure, digestive problems, and life-threatening complications years after infection.
Because many of these parasites have complex life cycles and survive inside human tissues, drug development has historically been slow. Older treatments such as eflornithine required intravenous administrations and hospital stays, making them hard to distribute in rural regions. Fexinidazole’s oral dosing changed the landscape dramatically by allowing treatment in community settings without specialized equipment.
Even so, scientists emphasize that understanding exact mechanisms of action is essential to improving existing drugs. The new study is the first to provide a direct link between fexinidazole’s action and specific molecular events inside the parasite, bringing much-needed clarity to how modern anti-trypanosome therapies work.
What the Study Reveals About Parasite Biology
One of the most interesting aspects of the research is its contribution to understanding how trypanosomes manage DNA replication. These parasites have unusual genomic architectures, including structures called kinetoplasts, which contain networks of circular DNA inside their mitochondria. The new findings suggest that fexinidazole-induced damage disrupts not just the main nucleus but potentially affects kinetoplast replication or maintenance as well. This is significant because kinetoplastid DNA is essential for parasite survival, and targeting it may prove to be an efficient strategy for future drug design.
The research also hints that the relationship between drug activation, metabolic stress, and ROS production is more nuanced than previously believed. Nitroaromatic drugs like fexinidazole are prodrugs, meaning they must be chemically activated inside the parasite. Variations in these activation pathways may influence how quickly DNA damage accumulates—and why some drugs in the same class behave differently.
Looking Ahead: Future Drug Development
The paper underscores that more work is needed to fully decode the cascade of molecular events that follows fexinidazole activation. While DNA damage and inhibited DNA synthesis are now known to be central, researchers still need to identify the exact intermediates and pathways involved. This will help determine how parasites might evolve resistance over time.
The Stony Brook team is continuing to explore how mitochondrial stress relates to drug resistance. Their work may identify new drug targets or help scientists modify existing drugs to make them more effective or longer lasting.
As parasitic infections continue to spread due to climate change, population movements, and shifting ecosystems, the need for accessible, safe, and effective treatments will only grow. Understanding the mechanisms behind current drugs is one of the most important steps in preparing for that future.
Research Paper:
Fexinidazole results in specific effects on DNA synthesis and DNA damage in the African trypanosome
https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0013647