New Research Shows the Inflammasome Drives Male-Specific Bone Loss in Gum Disease
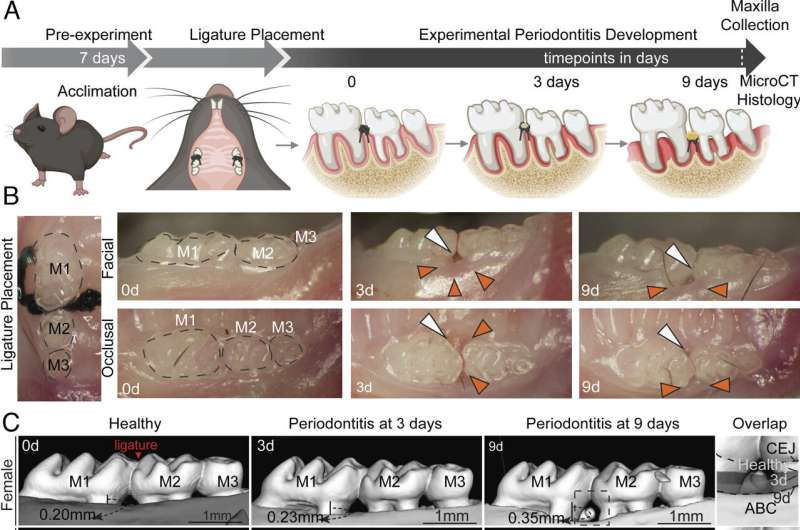
A new study from the University of North Carolina at Chapel Hill is shedding light on why periodontitis, a widespread gum disease that leads to inflammation and bone loss, tends to hit men harder than women. The researchers discovered that a key part of the immune system — the inflammasome — plays a sex-dependent role in driving bone destruction. What makes this especially interesting is that blocking this system appears to protect only males, not females, from gum-related bone loss.
This finding not only challenges long-held assumptions about how inflammation works across sexes but also opens the door to sex-specific treatments for a disease that affects millions worldwide.
What the Researchers Found
The team, led by scientists from the UNC Adams School of Dentistry and the UNC School of Medicine, analyzed more than 6,200 human samples gathered from three independent studies. They found that men consistently show higher levels of the inflammatory molecule IL-1β (interleukin-1 beta) in their gingival crevicular fluid — even when their gums are healthy. That same molecule becomes even more elevated during periodontitis.
Because IL-1β is closely tied to inflammation and bone breakdown, this suggests that men may naturally be more susceptible to inflammation-driven bone loss. The researchers describe this as a “male-biased” pattern of disease, something that hasn’t been fully appreciated before.
To explore this further, the team turned to controlled experiments in mice.
What the Mouse Studies Showed
In laboratory models, male mice displayed significantly greater IL-1β secretion in response to induced gum disease than female mice. This alone hinted at a sex-linked inflammatory difference, but the next steps made the picture even clearer.
When the inflammasome — the immune structure that activates IL-1β — was genetically deleted in mice, researchers saw a remarkable outcome:
Male mice experienced substantially less bone loss, while female mice showed no change at all.
The researchers then tried a drug-based approach by using a caspase-1/4 inhibitor, a pharmacological tool that blocks inflammasome activity. Again, the results were sex-specific:
- In male mice, the drug sharply reduced inflammatory cell infiltration and key signals that trigger osteoclastogenesis (the process that leads to bone resorption).
- In female mice, the drug had no measurable effect.
Taken together, these findings suggest the inflammasome has a critical, male-specific role in periodontal bone destruction.
The Role of the Male Reproductive System
One of the most intriguing parts of the research involved testing the influence of reproductive organs. The scientists removed the testes of male mice and the ovaries of female mice to see whether their hormonal environments were driving the sex difference.
The results were telling:
- Castrated male mice no longer responded to the inflammasome-blocking drug, meaning the bone-protective effects vanished.
- Female mice continued to show no response, regardless of whether they had ovaries.
This strongly suggests that male reproductive factors — possibly hormones, though more research is needed — are essential for inflammasome-driven inflammation in gum disease. Whatever drives the process in females appears to be different and unrelated to the inflammasome pathway.
What This Means for Gum Disease Treatment
This study is significant because it challenges the assumption that inflammatory diseases behave the same way across sexes. In fields like cardiology, endocrinology, and autoimmune disorders, sex-specific research is already expanding rapidly. Periodontitis now joins this growing list.
The researchers argue that sex-stratified therapies could offer better clinical outcomes. For example:
- Men might benefit from treatments that directly target the inflammasome or IL-1β, potentially preventing or reducing bone loss.
- Women may require therapies that target different inflammatory pathways, since blocking the inflammasome does not appear to help.
The study also underscores the need to understand gum disease in a more detailed biological context — not just in terms of hygiene habits, smoking, or other lifestyle factors. While behavior still plays a role, this research shows that biological differences between males and females are also at work.
A Quick Refresher: What Is the Inflammasome?
Since the inflammasome is central to this study, here’s a straightforward explanation.
The inflammasome is a protein complex inside certain immune cells. Its job is to detect harmful substances — things like bacteria, viruses, or cellular stress. When activated, it triggers a cascade that leads to the release of powerful inflammatory molecules, mainly IL-1β and IL-18.
This process can be protective, but when overactivated or improperly regulated, it contributes to chronic inflammation and tissue damage.
In the context of periodontitis, inflammasome activation drives bone-destroying processes around the teeth. The new study reveals that this destructive pathway is more active in males, which explains their higher susceptibility to severe gum disease.
Why Periodontitis Matters
Periodontitis isn’t just about bleeding gums or occasional discomfort. It’s a medically significant disease that can lead to:
- Loss of teeth
- Damage to supporting bone structures
- Chronic inflammation, which has been linked to heart disease, diabetes, and other systemic issues
More than that, it affects millions of adults, often progressing silently until considerable damage has occurred.
Understanding its biological drivers — particularly sex-linked ones — could reshape how dental professionals diagnose and treat patients.
The Importance of Sex-Specific Research
Historically, biomedical research leaned heavily toward male subjects, both in humans and animals. That has slowly changed over the past two decades, revealing important differences not only in hormones but also in immune responses, pain perception, and inflammation.
This new study adds to a growing body of evidence showing that men and women can experience the same disease through different biological mechanisms.
That’s why the findings have wide-ranging implications:
- They show the value of including balanced male and female subjects in research.
- They encourage the development of precision medicine, where treatment is tailored to a patient’s biological characteristics.
- They highlight potential blind spots in traditional models of inflammatory disease.
Gum disease may seem like a small corner of medicine, but the mechanisms at work are part of much larger biological systems.
What Comes Next?
The researchers emphasize that more work is needed, particularly in human trials. While mouse studies offer controlled environments and help uncover biological pathways, translating these findings into clinical practice requires testing in diverse human populations.
Future research will likely focus on:
- Identifying the exact male reproductive factors that enhance inflammasome activity
- Discovering alternative pathways in females that lead to bone loss
- Testing inflammasome-targeting drugs in human subjects
- Developing specific inflammatory markers that help clinicians assess risk based on sex
If successful, the outcome could be a new era of periodontal therapies with higher effectiveness and fewer side-effects, tailored uniquely for male and female patients.
Research Reference
PNAS – Inflammasome targeting for periodontitis prevention is sex dependent
https://www.pnas.org/doi/10.1073/pnas.2507092122