Northwestern Scientists Turn a Common Chemotherapy Drug into a 20,000-Times More Potent Cancer Killer
In a striking leap for cancer research, scientists at Northwestern University have restructured the well-known chemotherapy drug 5-fluorouracil (5-FU) into a far more powerful and safer form using nanomedicine technology. The result? A drug that is up to 20,000 times more effective at killing leukemia cells while leaving healthy tissues unharmed — at least in animal tests.
Transforming a Familiar Drug with Nanotechnology
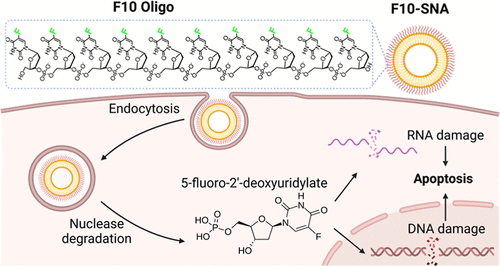
The Northwestern team, led by Professor Chad A. Mirkin, redesigned 5-FU into what’s known as a Spherical Nucleic Acid (SNA). SNAs are tiny, globe-shaped nanostructures made up of a core nanoparticle surrounded by a dense shell of DNA or RNA strands. This structure isn’t random — it changes how the drug interacts with cells in the body.
Traditional 5-FU is known for its poor solubility — less than 1 percent of it dissolves properly in biological fluids. Because of this, the body can’t absorb it efficiently, and it often fails to reach cancer cells in effective doses. At the same time, the little that does circulate can damage healthy tissue, causing harsh side effects such as nausea, fatigue, and even heart complications in rare cases.
To overcome this, Mirkin’s team chemically integrated 5-FU molecules directly into the DNA shell of the SNA rather than simply coating or encapsulating them. This fundamental shift in structure made the drug more soluble, more stable, and more easily recognized by cancer cells.
Targeting Leukemia Cells with Precision
The researchers tested this redesigned drug on acute myeloid leukemia (AML) — one of the most aggressive and difficult-to-treat blood cancers. AML cells are known to have high levels of scavenger receptors on their surfaces, which naturally pull in SNA structures. This means the SNA-form of 5-FU can enter leukemia cells without needing to be forced inside. Once inside, enzymes in the cell break down the DNA shell, releasing the active drug directly into the cancer cell.
Compared to traditional chemotherapy, the results were dramatic:
- 12.5 times higher cell uptake
- Up to 20,000 times greater cell-killing power
- 59-fold reduction in cancer progression
- No detectable side effects in mice
In essence, the SNA form of 5-FU could wipe out leukemia cells in the blood and spleen of treated mice, nearly eliminating the cancer while sparing normal tissues. For the animals, it also meant a significant extension in survival.
What Makes This Different: Structural Nanomedicine
This breakthrough highlights a new and fast-growing area called structural nanomedicine. Unlike conventional drug design, which focuses mainly on what molecules are made of, structural nanomedicine focuses on how those molecules are arranged in three-dimensional space. The shape and architecture of the drug delivery system — not just its chemical composition — determines how it interacts with the body.
By designing a precise 3D structure, scientists can dictate how the drug enters cells, where it goes in the body, how long it stays active, and how efficiently it performs its function. In this case, changing 5-FU from a small, free-floating molecule into a DNA-based spherical structure completely transformed its behavior.
SNAs, originally developed by Mirkin’s lab, are already the foundation for seven clinical trials targeting cancers, autoimmune conditions, and even neurological diseases. This latest achievement suggests SNAs could become a universal platform for reengineering existing drugs, making them more effective and less toxic.
From Lab to Future Therapies
While these findings are still pre-clinical, they offer one of the clearest examples yet of how nanomedicine can reshape the way chemotherapy works. The team now plans to conduct larger small-animal studies, followed by larger animal models, and eventually move into human clinical trials, pending funding and regulatory approval.
If this approach translates successfully to humans, it could mean more effective chemotherapy treatments with fewer side effects — one of the biggest goals in modern cancer therapy. Instead of flooding the body with high doses of toxic chemicals, future chemotherapies could be finely tuned and structurally engineered to seek out cancer cells while ignoring everything else.
A Closer Look at Spherical Nucleic Acids (SNAs)
To understand how powerful this approach is, it helps to look at SNAs themselves. These structures consist of:
- A core (often a nanoparticle of gold, silica, or another inert material)
- A shell of tightly packed DNA or RNA strands
- A radial arrangement that lets them enter cells efficiently
Because of this structure, SNAs are recognized by cell receptors far more readily than linear DNA or free drug molecules. This makes them useful not just in chemotherapy, but also in gene regulation, vaccine development, and immunotherapy.
Moreover, SNAs can be tailored to target specific cell types. By adjusting the DNA or RNA sequences, researchers can guide them toward certain tissues or diseases. The new SNA-5-FU hybrid takes advantage of this property to home in on AML cells while bypassing healthy tissue.
Why Poor Solubility Is a Big Deal in Medicine
The poor solubility of many chemotherapy drugs is one of the biggest hurdles in effective cancer treatment. A drug that doesn’t dissolve properly in blood can’t circulate efficiently and often gets cleared from the body before reaching tumor cells. To compensate, doctors usually administer high doses, increasing the risk of toxicity.
By redesigning 5-FU into an SNA form, researchers solved this core issue. The DNA-based outer shell acts as a solubilizing and targeting system, keeping the drug stable in biological fluids while directing it toward the right cells. This strategy could be applied to other poorly soluble drugs, opening the door to reviving older chemotherapies that were once limited by delivery problems.
Broader Implications for Cancer Research
This discovery represents more than just an upgrade to one drug. It’s a blueprint for reimagining how existing medications can be re-engineered at the nanoscale. If chemotherapies can be rebuilt as SNAs, similar approaches could transform treatments for:
- Solid tumors such as breast, colon, and pancreatic cancers
- Autoimmune disorders, where precise immune modulation is needed
- Infectious diseases by creating SNA-based vaccines
- Neurodegenerative diseases like Alzheimer’s, through targeted delivery across the blood-brain barrier
Each of these applications benefits from the same principle — that changing a molecule’s structure can change its destiny inside the body.
The Road Ahead
While the excitement is justified, researchers are cautious. Animal models don’t always predict human outcomes, and challenges remain around manufacturing, stability, cost, and long-term safety. The team must prove that these SNA-based drugs can be produced reliably, remain stable over time, and don’t accumulate or trigger immune reactions in the body.
Still, Mirkin’s work marks a turning point. It shows that rethinking the physical design of drugs can yield breathtaking improvements without inventing entirely new molecules. This structural revolution could one day make chemotherapy smarter, gentler, and dramatically more effective.
Research Reference:
Chemotherapeutic Spherical Nucleic Acids – ACS Nano (2025)