Prebiotic Diet Shows Promise in Reducing Impulsivity After Traumatic Brain Injury in Rats

A new study from researchers at The Ohio State University offers early but intriguing evidence that a dietary prebiotic may help reduce one of the most stubborn behavioral problems that can follow a traumatic brain injury (TBI): increased impulsivity. This finding comes from carefully controlled experiments in rats, and while it is far from a clinical treatment for humans, it adds weight to the growing body of research linking the gut microbiome to brain function and long-term recovery after injury.
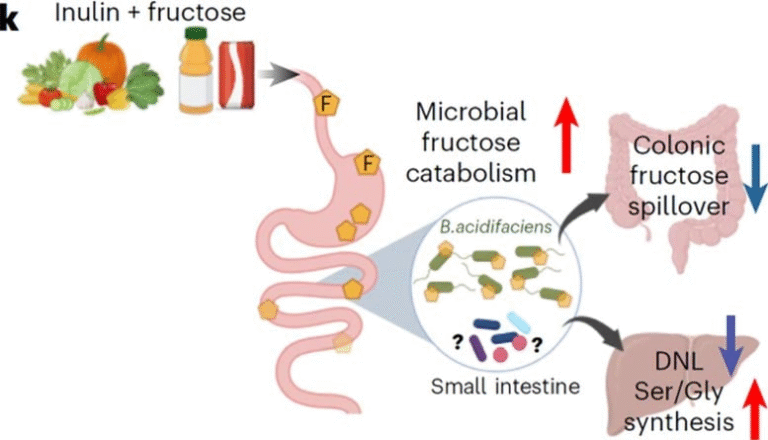
The researchers focused on galacto-oligosaccharide (GOS), a well-known prebiotic that supports the growth of beneficial gut bacteria. Their goal was to see whether influencing gut health before and after a moderate brain injury could mitigate the chronic decision-making deficits and impulse-control problems often reported in both humans and animal models of TBI. Importantly, this work builds on their earlier findings showing that TBI tends to disrupt the gut microbiome, and those disruptions strongly correlate with long-term behavioral issues.
In the study, rats were fed either a standard diet or a diet containing 2% GOS beginning six weeks before injury. The supplemented diet continued for 60 days after the injury as well. The injury itself was designed to mimic the kind of moderate TBI humans may experience in events like car accidents or falls. After recovery, the rats underwent a series of behavioral tests designed to evaluate anxiety-like behavior, depressive-like tendencies, learning, memory, and impulsivity.
One of the most revealing tests was a rat version of a gambling task, a decision-making challenge where rats choose between four options that dispense different amounts of sugar pellets. To succeed, the animals need to wait for a cue light before acting—something that becomes significantly harder after TBI. As expected, the injured rats on a normal diet showed marked impairments in impulse control. However, those that had been consuming the GOS-supplemented diet showed noticeably better impulse regulation than their counterparts. The improvement was not complete—they were still more impulsive than uninjured rats—but it was a clear, measurable reduction, one of the most encouraging outcomes the team has seen after testing many potential interventions.
The findings were presented at Neuroscience 2025, the annual meeting of the Society for Neuroscience. The study’s senior author, Cole Vonder Haar, explained that long-term impulsivity after TBI is notoriously difficult to treat and that even modest improvements are meaningful. Since there are currently no FDA-approved treatments specifically targeting TBI itself, individuals with lingering symptoms often rely on rehabilitation or medications that only address secondary issues such as depression or anxiety. This is why gut-based interventions are generating attention—they might offer a new pathway to improving quality of life.
Gut Dysbiosis and TBI: Why the Microbiome Matters
In recent years, scientists have increasingly recognized that TBI affects far more than the brain. Moderate brain injuries frequently lead to gut dysbiosis, a disruption of the microbial community in the digestive tract. This can trigger inflammation, alter metabolism, and interfere with neurochemical pathways that link the gut and brain. Previous work from the same research group showed that changes in gut bacteria shortly after injury could predict long-term impairments in decision-making months later. But correlation does not equal causation, which is why the new intervention-based study is important—it tests whether deliberately modifying the microbiome can alter behavioral outcomes.
This gut-brain relationship is part of what scientists call the gut-brain axis, a bidirectional communication system involving neural, hormonal, and immune pathways. When the gut is disrupted, it can send stress signals to the brain, potentially affecting behavior, cognition, and mood. When the brain is injured, signals from the nervous system can disrupt the gut’s environment as well. This feedback loop means interventions could theoretically work from either end.
Details of the Experimental Design
The controlled design of the experiment is key, so here are the specifics:
- Rats received either a control diet or 2% GOS-supplemented diet.
- The diet began six weeks before the TBI to establish gut microbiome changes prior to injury.
- The diet continued 60 days after the TBI to assess long-term effects.
- Behavioral tests included anxiety-like behavior, depressive-like behavior, learning, memory, impulsivity, and decision-making.
- The rodent gambling task was central to evaluating impulsivity.
- Rats with TBI on normal diet showed long-term chronic impairments in decision-making.
- Rats with TBI on the GOS diet showed reduced impulsivity compared to injured rats without GOS.
These results do not suggest that diet alone can cure TBI-related problems, but they do indicate that microbiome-targeted strategies might meaningfully reduce certain symptoms.
Why Impulsivity Matters After TBI
Impulsivity isn’t a minor issue. In people who have experienced a moderate or severe brain injury, impaired impulse control can interfere with employment, relationships, financial stability, and daily decision-making. Chronic issues with impulsivity and risk-taking behavior are among the most commonly reported—and hardest to treat—aftereffects. Interventions that lessen impulsivity even slightly could help people regain autonomy and improve long-term wellbeing.
Ongoing Research and Future Directions
Vonder Haar also chaired a minisymposium at the same conference specifically about the gut microbiome’s role in traumatic brain injury. The researchers published a 2025 review in The Journal of Neuroscience summarizing existing evidence supporting the gut as a modulator of TBI pathology. This review highlights mechanisms such as immune activation, gut permeability, metabolic shifts, and possible disruptions to neural communication.
The broader hope is that by understanding these mechanisms, researchers will eventually be able to identify which microbial changes are harmful, which are helpful, and how to target them safely. Many questions remain:
Which gut bacteria change after TBI?
Which microbial metabolites influence impulsivity or cognition?
Could probiotics or other prebiotics have stronger effects than GOS?
Is there an optimal timeframe for microbiome-based treatments after injury?
For now, the answer is that this research is still early—but promising.
Extra Context: What Are Prebiotics and Why GOS?
Prebiotics are nondigestible fibers that nourish beneficial bacteria in the gut. Unlike probiotics, which introduce live bacteria, prebiotics act as food for the microbes already living in your digestive system. GOS, the prebiotic used in this study, naturally occurs in some dairy products and is commonly added to foods or supplements aiming to promote gut health.
Studies in humans have shown that GOS can support the growth of Bifidobacteria, a group of microbes linked to positive immune and metabolic outcomes. GOS is considered safe, widely available, and inexpensive, which makes it an appealing candidate for future clinical research in TBI recovery.
Extra Context: Understanding Traumatic Brain Injury
A moderate traumatic brain injury typically involves a significant blow or jolt to the head, often causing loss of consciousness. The Brain Injury Association of America reports nearly 3 million TBI-related emergency visits each year in the United States. More than 11 million Americans over age 40 who have experienced a TBI with loss of consciousness now live with long-term disability. Because there are no direct treatments for the injury itself, ongoing research looks for ways to help people cope with or reduce chronic symptoms.
The new findings do not change medical practice, but they contribute a new angle to an active field—one that may ultimately reshape how we think about recovery.
Research Reference
The Gut Microbiome as a Modulator of Traumatic Brain Injury Pathology and Symptoms (2025)
https://doi.org/10.1523/jneurosci.1337-25.2025