Rare POLE Gene Mutation Strongly Linked to Exceptional Immunotherapy Response in Colorectal Cancer

A new wave of research is shedding light on a very specific and rare type of genetic mutation that may dramatically improve how we treat metastatic colorectal cancer. Scientists at The University of Texas MD Anderson Cancer Center have identified a subset of POLE gene mutations, known as loss-of-proofreading (LOP) mutations, that appear to predict remarkably strong and durable responses to immunotherapy in colorectal cancer patients. While POLE mutations have been discussed for years as potential biomarkers, this study finally draws a clear distinction between which POLE mutations truly matter for immunotherapy response and which do not.
This is a major insight because, until now, all POLE mutations were often lumped together in clinical interpretation. In practice, that meant patients who were unlikely to benefit from immunotherapy might still receive it, while those with the right mutation subtype were not always identified early enough. The new findings give a sharper, more clinically relevant picture.
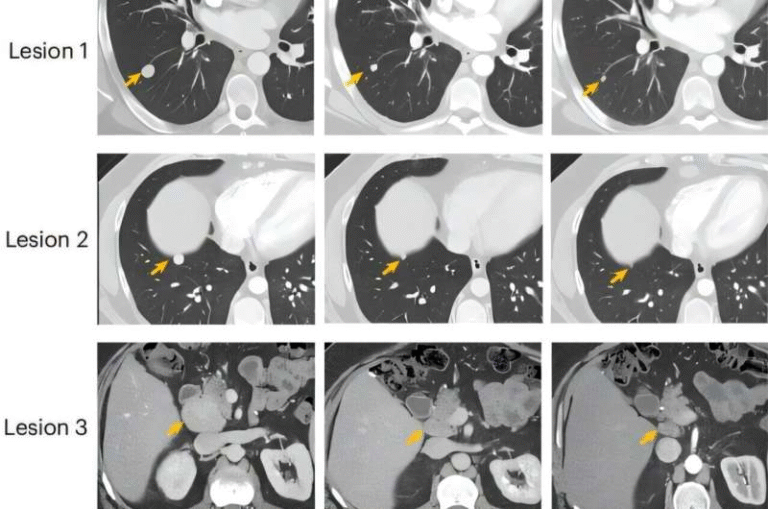
Researchers retrospectively examined a massive dataset of 69,223 tumor samples, representing many cancer types, using cBioPortal. Out of these, 2.8% showed some form of POLE mutation. However, only 0.1% carried the specific LOP variants that appear to be biologically meaningful for immunotherapy. In a clinical cohort of 11 metastatic colorectal cancer patients treated at MD Anderson, nine carried LOP POLE mutations. Their responses to immune checkpoint therapy were stunning: an 88.9% objective response rate and a 100% disease control rate. Tumors consistently shrank, and patients maintained durable benefit over time.
By contrast, patients with non-LOP POLE mutations—despite still having a POLE alteration—did not experience the same benefits. This is a crucial difference because it clarifies that not all POLE mutations act the same way biologically. The LOP subtype specifically disrupts the proofreading ability of the POLE protein, leading to extremely high mutation rates in tumor cells. This “hypermutated” state appears to make tumors more visible to the immune system and more responsive to immunotherapy.
What makes this discovery even more meaningful is how it can impact real-world patient care. If clinicians can determine whether a patient carries a LOP POLE mutation, they can make better-targeted decisions about whether immunotherapy should be used. Those with LOP POLE tumors may benefit far more from immunotherapy than from standard chemotherapy. Meanwhile, patients with non-LOP mutations can avoid therapies unlikely to help them, sparing them side effects and helping guide them toward more suitable treatments.
The study also reinforces the value of broad tumor sequencing. Colorectal cancer patients, even though LOP POLE mutations are rare, may benefit significantly from genomic testing. Identifying this mutation early could completely change a patient’s treatment plan.
Below, you’ll find extra context about POLE, immunotherapy, and how this fits into the broader field of cancer genetics, so readers can better understand the importance of these findings.
What the POLE Gene Does
The POLE gene provides instructions for producing the catalytic subunit of DNA polymerase epsilon, a major enzyme involved in DNA replication. Importantly, it contains a proofreading function that detects and fixes errors during replication. When this proofreading ability is impaired due to a loss-of-proofreading mutation, the cell accumulates mutations at a much higher rate than normal. The resulting hypermutated environment can make the cancer cells stand out to the immune system.
Not every POLE mutation disrupts proofreading. That’s the critical point highlighted by this new study. Some mutations alter other parts of the protein without meaningful impact on error correction and thus do not increase tumor mutational burden in the same way. Only LOP mutations, which specifically damage exonuclease-mediated proofreading, appear tied to exceptional immunotherapy responses.
Why Hypermutated Tumors Respond Better to Immunotherapy
Immune checkpoint inhibitors work by helping the immune system recognize cancer cells. Tumors with very high numbers of mutations tend to produce more neoantigens, which are abnormal proteins the immune system can detect. The more neoantigens, the easier it becomes for immune cells to find and attack the tumor after checkpoint inhibition.
Tumors that are hypermutated because of LOP POLE mutations behave similarly to cancers with microsatellite instability-high (MSI-H) status, which are already known to respond well to immunotherapy. But the key difference is that LOP POLE tumors can be microsatellite-stable (MSS) yet still show strong response, expanding the group of patients who could benefit from immunotherapy.
How Rare Are LOP POLE Mutations?
In the pan-cancer dataset evaluated, only about 0.1% of tumors carried LOP POLE mutations. In colorectal cancer specifically, the percentage is slightly higher but still very rare. However, rarity does not diminish clinical importance. In fact, rare but highly predictive biomarkers are extremely valuable because they let clinicians tailor therapy with high confidence.
Even a fraction of a percent matters when the predictive power is this strong.
Implications for Colorectal Cancer Treatment
This discovery has several possible implications:
1. Better patient selection for immunotherapy.
Instead of treating all POLE-mutated patients the same way, oncologists can now distinguish between LOP and non-LOP variants.
2. Avoiding ineffective therapy for non-LOP patients.
Non-LOP POLE mutations are not linked to high response rates. Knowing this helps prevent wasted time and toxicity.
3. Potential shift in treatment sequencing.
Patients with LOP POLE mutations might benefit from immunotherapy earlier, potentially even before chemotherapy.
4. Stronger justification for comprehensive tumor sequencing.
Identifying a rare but highly actionable mutation underscores the need for modern molecular diagnostics.
5. Improved clinical trial design.
Future trials can stratify patients more accurately, leading to clearer results and better-targeted therapies.
Broader Context: POLE Mutations in Cancer Research
Researchers have been interested in POLE mutations for years. Some early studies hinted that these mutations might predict better outcomes, but results were inconsistent because they did not distinguish LOP from non-LOP variants. This new research resolves much of that confusion by offering a refined classification system.
The study also highlights how advancing sequencing technology enables better precision medicine. Earlier oncology depended on broad categories; modern oncology is increasingly about pinpointing molecular features that make each tumor unique.
Key Takeaway
The discovery that LOP POLE mutations are strongly associated with exceptional immunotherapy responses in metastatic colorectal cancer is a meaningful step forward in personalized oncology. These mutations are rare, but when present, they can dramatically shape treatment outcomes. By improving how POLE variants are classified and understood, the study offers a clearer path for selecting the right therapies for the right patients.
This reinforces the idea that precision medicine is not just about finding common biomarkers—it’s also about identifying rare but powerful ones that can transform individual patient outcomes.
Research Paper:
Prognosis and treatment response stratification according to loss of proofreading (LOP) POLE variants
https://jitc.bmj.com/content/13/11/e012190