Scientists Discover Bacterial Molecule That Supercharges Chemotherapy
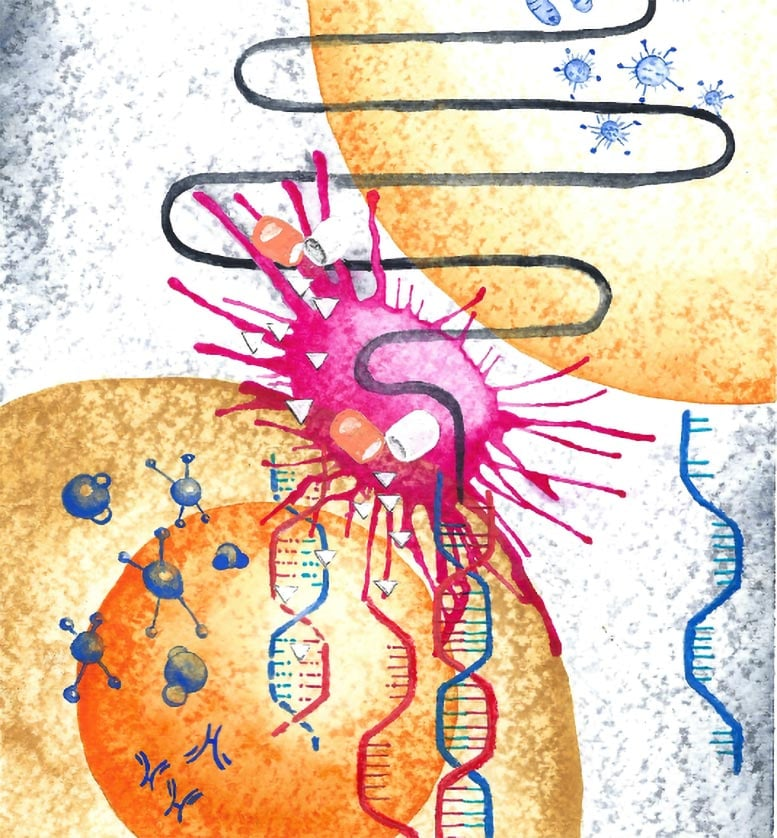
A team of international researchers has made an exciting discovery: bacteria living inside tumors can produce a molecule that strengthens the power of chemotherapy drugs. This finding could open the door to creating new, more effective cancer treatments, where microbes themselves—or synthetic versions of their molecules—become allies in the fight against cancer.
The study was carried out by scientists at the MRC Laboratory of Medical Sciences (LMS) in London, Imperial College London, and the University of Cologne, along with collaborators across Europe. Their research was recently published in Cell Systems and is already drawing attention for its potential to reshape how we think about both cancer and the microbes that live inside our bodies.
Tumors Host Their Own Microbial Communities
Most of us know that bacteria thrive in our gut, skin, and mouth, where they influence digestion, immunity, and even mental health. What’s less well known is that tumors also host microbial communities. These bacteria live in and around cancer cells, but their roles have remained mysterious. Are they harmful passengers, neutral residents, or active participants in how tumors grow and respond to treatment?
This research team set out to understand exactly that: what are these bacteria doing inside tumors, and could they be leveraged for therapy?
Screening Reveals a Surprising Molecule
The scientists performed an extensive screening process, testing more than 1,100 different conditions using the microscopic worm Caenorhabditis elegans as a model. This tiny worm is a popular research organism for studying host–microbe interactions because it’s simple, transparent, and easy to manipulate.
Through these tests, the team discovered that Escherichia coli (E. coli), a bacterium often found in the gut, produced a small molecule called 2-methylisocitrate (2-MiCit). What makes 2-MiCit remarkable is that it boosts the effectiveness of the widely used chemotherapy drug 5-fluorouracil (5-FU).
This was the first clue that bacteria might not just coexist in tumors, but actually contribute to the way cancer responds to drugs.
How 2-MiCit Works in Cancer Cells
Once 2-MiCit was identified, the researchers wanted to know how it interacts with cancer cells. They discovered that the molecule disrupts a key enzyme inside mitochondria, which are the energy powerhouses of cells.
When this enzyme is blocked, cancer cells suffer from:
- Energy stress inside the mitochondria
- DNA damage, making it harder for them to divide and survive
- Activation of pathways that slow cancer progression
This combination creates a multi-pronged attack. On its own, 2-MiCit harms cancer cells, but when paired with 5-FU, the effects are amplified. The chemotherapy drug 5-FU interferes with DNA synthesis, and with 2-MiCit weakening the cancer cell’s metabolism, the drug becomes much more lethal to tumor cells.
Testing in Multiple Models
To be sure this wasn’t just a fluke in worms, the team moved on to more advanced systems:
- Human cancer cell cultures: 2-MiCit showed strong anti-cancer activity in the lab.
- Fruit fly model (Drosophila): In flies engineered to develop colorectal tumors, the molecule reduced tumor growth and even extended lifespan.
Both models confirmed the same story: this bacterial metabolite has real therapeutic potential.
From Natural Molecule to Synthetic Drug
Natural molecules are often a starting point for drug development. In this case, the researchers collaborated with medicinal chemists to make a synthetic version of 2-MiCit. By tweaking the structure, they created a compound that was even more potent at killing cancer cells than the original bacterial product.
This is an exciting proof of concept: by studying microbes in tumors, scientists can find useful natural compounds and then optimize them into stronger drugs.
Why This Matters for Cancer Treatment
This discovery has several important implications:
- New cancer drug possibilities: 2-MiCit and its derivatives could inspire drugs that work alongside chemotherapy to increase its success.
- Personalized medicine: Not all tumors harbor the same bacteria. Understanding a patient’s tumor microbiome could help doctors predict how well chemotherapy will work, or which treatments to combine.
- Changing how we see microbes: Bacteria inside the body aren’t just harmful invaders or passive bystanders. Some can actively help fight disease.
Funding and Collaboration
The project was supported by major funding bodies, including:
- The Leverhulme Trust
- The Wellcome Trust/Royal Society
- The German Research Foundation (DFG)
- The UK Medical Research Council (MRC)
The research brought together microbiologists, cancer biologists, chemists, and computational modelers. This multidisciplinary approach was key in moving from worm experiments to computer models, cell culture, and animal testing.
Limitations and Open Questions
While the discovery is promising, it’s important to highlight that the findings are still preclinical:
- No human trials yet: The effects of 2-MiCit haven’t been tested in cancer patients.
- Dosing challenges: We don’t yet know how much of the molecule tumors naturally produce, or how a drug version would behave in the human body.
- Safety concerns: Any new molecule needs to be checked for side effects in healthy cells and tissues.
- Tumor variability: Not every tumor has the same bacterial residents. This means some patients may benefit more than others.
Despite these uncertainties, the discovery is an exciting step forward in microbiome-cancer research.
Extra Knowledge: The Tumor Microbiome
To give readers more context, let’s explore a broader question: what is the tumor microbiome, and why does it matter?
What Is the Tumor Microbiome?
The tumor microbiome refers to the collection of bacteria, fungi, and other microbes that live inside tumors. For a long time, the idea of bacteria existing in tumors was controversial, but advances in sequencing technology have revealed that many cancers host their own tiny ecosystems.
How Can Microbes Influence Cancer?
Microbes inside tumors can:
- Promote cancer growth by triggering inflammation
- Metabolize drugs, making them less effective
- Support anti-cancer activity, as shown in this new study
For example, in 2021, researchers discovered that certain bacteria inside pancreatic tumors could metabolize gemcitabine, a chemotherapy drug, rendering it ineffective. That finding highlighted the dark side of microbes in tumors. Now, with 2-MiCit, we see the bright side: a bacterial molecule that helps treatment instead of hindering it.
Extra Knowledge: 5-Fluorouracil (5-FU)
Since this study revolves around boosting the power of 5-FU, let’s briefly look at what this drug is.
What Is 5-FU?
5-Fluorouracil is one of the oldest and most commonly used chemotherapy drugs. It has been in use since the 1950s and is a cornerstone in treating colorectal, breast, head and neck, and other cancers.
How Does It Work?
5-FU interferes with the synthesis of DNA and RNA. By mimicking normal molecules used in DNA production, it tricks cancer cells and disrupts their ability to replicate.
Challenges with 5-FU
- Side effects: Because it affects both healthy and cancerous cells, patients often experience nausea, mouth sores, and low blood counts.
- Resistance: Some tumors evolve ways to resist 5-FU, making it less effective over time.
This is why a molecule like 2-MiCit is so exciting—it has the potential to make 5-FU work better, possibly at lower doses, reducing side effects while increasing effectiveness.
Looking Ahead
This discovery suggests a new strategy in cancer therapy: instead of targeting just the tumor, researchers can target the tumor and its microbial environment together. If 2-MiCit or its synthetic versions make it to clinical trials and prove safe and effective, they could represent an entirely new class of microbiome-inspired cancer drugs.
It also emphasizes the need for personalized treatment that looks not only at the patient’s genetics and tumor biology but also at their microbes.
Research Reference
Chemotherapy modulation by a cancer-associated microbiota metabolite – Cell Systems (2025)