Scientists Identify a Little-Known Enzyme That Could Unlock New Treatments for Neurodegenerative Diseases
Researchers have identified a previously underappreciated enzyme that may play a central role in myelin damage, a process linked to several serious neurological conditions including multiple sclerosis, Alzheimer’s disease, stroke, and traumatic brain injury. The enzyme, known as Cell Migration Inducing and Hyaluronan-Binding Protein (CEMIP), has emerged as a potential new target for therapies aimed at slowing or even reversing damage to the nervous system.
The findings come from a detailed study conducted by scientists at Oregon Health & Science University (OHSU) and published in the peer-reviewed journal ASN Neuro in 2025. Using a combination of cell cultures, animal models, and human post-mortem tissue, the researchers traced how CEMIP interferes with the brain’s ability to repair itself after injury.
What Myelin Is and Why It Matters
To understand the importance of this discovery, it helps to understand myelin. Myelin is a fatty, insulating layer that wraps around nerve fibers, known as axons. Its main job is to speed up electrical signal transmission between nerve cells, allowing the brain and spinal cord to communicate efficiently.
When myelin is damaged, nerve signals slow down or fail entirely. This breakdown is a defining feature of multiple sclerosis, but it also occurs after strokes, brain injuries, and in certain forms of dementia, including Alzheimer’s disease. In many cases, the body tries to repair myelin, but this repair process often stalls or fails.
The Role of CEMIP in Myelin Breakdown
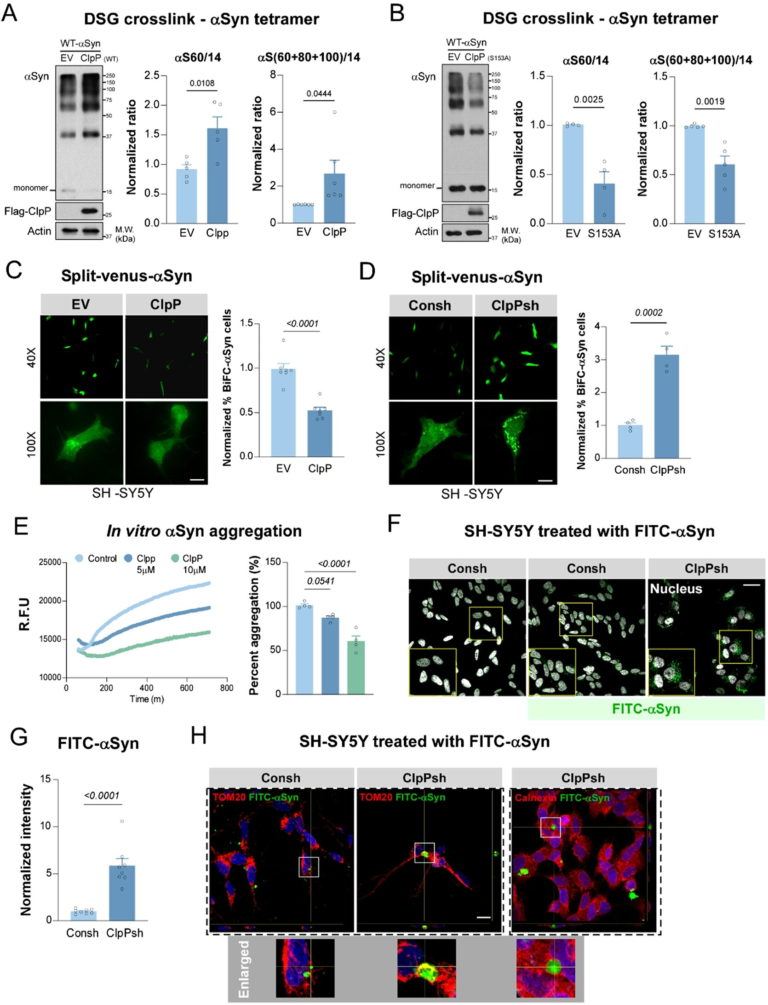
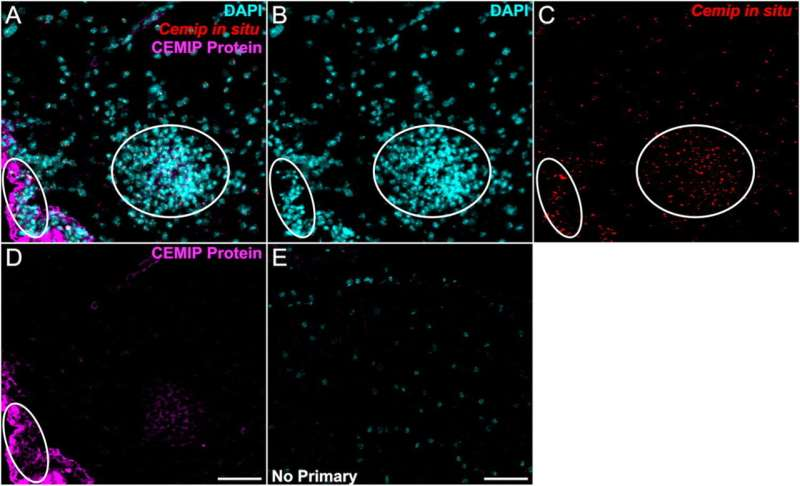
The OHSU researchers discovered that CEMIP becomes highly active in areas of the brain and spinal cord where myelin is damaged. CEMIP’s main biochemical function is to break down hyaluronic acid, a molecule that accumulates in brain tissue following injury or inflammation.
Hyaluronic acid itself is not harmful. In fact, it plays an important role in tissue structure and repair. However, when CEMIP breaks it down, it produces small hyaluronic acid fragments that turn out to be problematic.
These fragments interfere with the normal repair process by blocking the maturation of oligodendrocyte progenitor cells. These progenitor cells are essential because they develop into oligodendrocytes, the specialized cells responsible for producing new myelin. When this maturation process is disrupted, myelin repair grinds to a halt.
Evidence from Cells, Mice, and Human Tissue
The strength of this study lies in how broadly the researchers tested their hypothesis.
In cell culture experiments, elevated levels of CEMIP were shown to prevent oligodendrocyte progenitor cells from fully maturing. In mouse models, particularly those with experimental autoimmune encephalomyelitis (EAE), a commonly used model of multiple sclerosis, CEMIP levels increased sharply in regions undergoing active myelin damage.
The researchers also examined human tissue donated by people with multiple sclerosis. In these samples, CEMIP was found in high concentrations within brain lesions where myelin had been lost. This confirmed that the enzyme is not just a laboratory artifact, but is actively involved in human disease.
Advanced imaging techniques, including immunostaining and RNA in situ hybridization, revealed that CEMIP was localized within cells associated with inflammation and tissue damage. Areas with elevated CEMIP also showed increased cellular density, consistent with immune cell infiltration during disease progression.
Why the Brain Might Use CEMIP at All
From an evolutionary standpoint, CEMIP is not inherently “bad.” Researchers believe it plays a useful role in the early stages of injury response. When tissue is damaged, breaking down excess hyaluronic acid may help regulate inflammation and clear debris.
The problem arises when this response becomes chronic. In long-lasting neurological diseases, CEMIP remains active for too long, continually generating hyaluronic acid fragments that prevent long-term repair. In aging brains, where hyaluronic acid naturally accumulates, this effect may be even more pronounced.
In short, CEMIP appears to be helpful in short bursts but harmful when left unchecked.
Links to Multiple Neurological Conditions
While the study focused heavily on multiple sclerosis, the implications extend far beyond a single disease.
Myelin disruption is increasingly recognized as a contributing factor in Alzheimer’s disease, where white matter damage can precede cognitive symptoms. Similarly, stroke survivors and people with traumatic brain injuries often experience long-term neurological deficits linked to incomplete myelin repair.
Because CEMIP operates at a fundamental level of myelin regeneration, targeting it could have broad therapeutic value across many conditions where nerve insulation is compromised.
A Potential Path Toward New Treatments
One of the most encouraging aspects of this research is that CEMIP may be druggable. Previous work at OHSU identified a natural compound derived from dahlias that can inhibit CEMIP activity. While this compound is still far from clinical use, it provides proof that the enzyme can be targeted.
The new study strengthens the case that CEMIP is the right target to pursue, showing that reducing its activity could restore the brain’s ability to generate new myelin. Future research will focus on refining inhibitors, testing safety, and determining whether CEMIP levels could serve as a biomarker for disease progression or treatment response.
What This Means for the Future of Neurodegenerative Research
For decades, treatments for diseases like multiple sclerosis have focused primarily on suppressing the immune system. While these therapies reduce inflammation, they often do little to repair existing damage. A strategy centered on promoting myelin repair represents a major shift in thinking.
By identifying CEMIP as a key molecular roadblock to regeneration, this research opens the door to therapies that don’t just slow disease, but actively help the nervous system heal itself.
About the Research Team
The study was led by neuroscientists at OHSU, with senior leadership from Larry Sherman, Ph.D., a professor in the Division of Neuroscience at the Oregon National Primate Research Center. The research team included Alex Peters, Kanon Yasuhara, Weiping Su, Steven Matsumoto, Peter Pham, Fatima Banine, Eliana Harris, and Stephen A. Back, bringing together expertise in neuroscience, molecular biology, and neuropathology.
Research Reference:
Here – https://doi.org/10.1080/17590914.2025.2600157