Scientists Uncover How Cancer Cells Power Up Under Pressure
Cancer research has taken an intriguing new turn. A team at the Centre for Genomic Regulation (CRG) in Barcelona has discovered a hidden mechanism that helps cancer cells survive under extreme physical stress. The findings, published in Nature Communications in July 2025, shed light on how cells generate quick bursts of energy to repair DNA damage and endure mechanical challenges inside the body.
This is not just another study about cancer metabolism. It reveals something fundamentally new: when cancer cells are physically squeezed, they trigger their mitochondria — the well-known powerhouses of the cell — to rush toward the nucleus and pump out extra ATP (adenosine triphosphate). This immediate energy delivery helps repair broken DNA strands and keeps the cells alive in tough conditions.
The Discovery in Detail
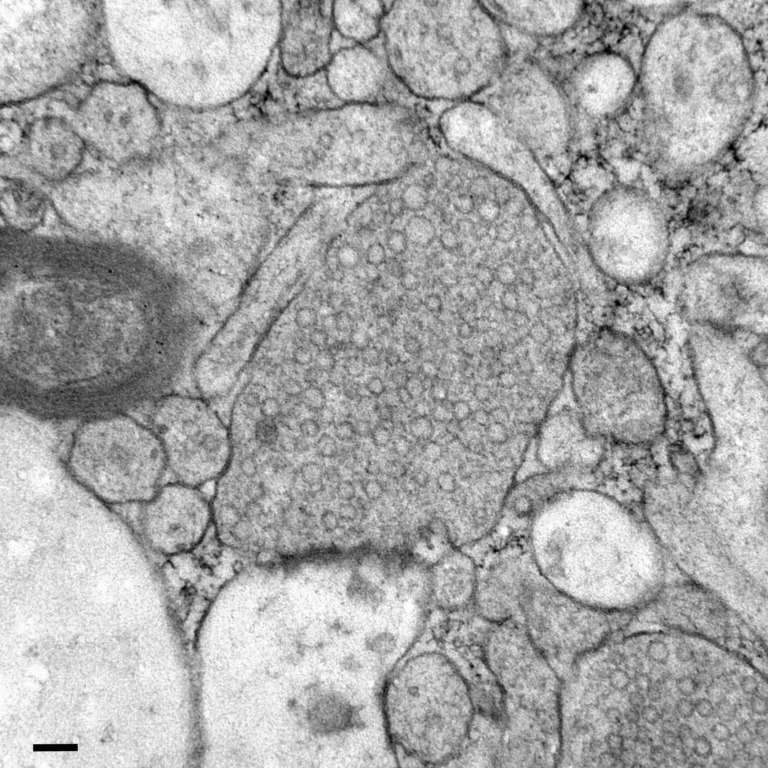
Researchers at CRG used a specialized microscope that can physically compress living cells down to about three microns in width — roughly one-thirtieth the thickness of a human hair. When they squeezed HeLa cells (a commonly studied cancer cell line), they noticed a remarkable reaction.
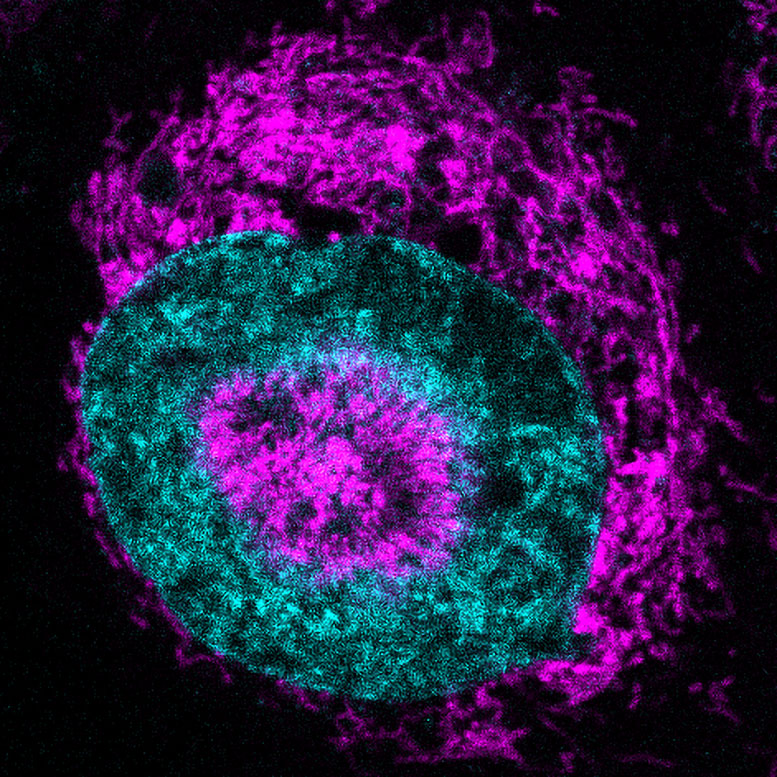
Within seconds of compression, mitochondria moved to surround the nucleus, forming a halo-like structure. This tight clustering around the nuclear envelope was so pronounced that the nucleus itself developed small dimples where mitochondria pressed against it.
The researchers gave this structure a name: NAMs (nucleus-associated mitochondria). The phenomenon was observed in about 84 percent of confined HeLa cells, compared to virtually none in uncompressed cells.
To test whether these NAMs were more than just a structural oddity, the scientists used a fluorescent sensor that glows when ATP enters the nucleus. The results were striking — nuclear ATP levels shot up by about 60 percent within three seconds of squeezing. This showed that NAMs were not just clustering, but actively delivering energy to the nucleus.
Why the Energy Boost Matters
Physical compression of cells isn’t harmless. It causes DNA stress, snapping strands and tangling chromatin (the tightly packed form of DNA). For cells to keep dividing, they must quickly repair this kind of damage.
DNA repair requires a lot of energy. By clustering mitochondria around the nucleus, cancer cells essentially create an emergency energy supply line, ensuring repair crews have enough ATP to untangle DNA and fix breaks.
In experiments, squeezed cells with NAM-driven ATP surges managed to repair DNA within hours. Cells without this energy boost, however, struggled — they stopped dividing properly and accumulated genomic errors.
Evidence from Real Tumor Samples
It’s one thing to observe this in lab-grown cells, but does it also happen in actual tumors inside the body? To find out, the researchers analyzed breast tumor biopsies from 17 patients.
Indeed, NAM halos were visible in clinical samples. They appeared in 5.4 percent of nuclei at invasive tumor edges compared to only 1.8 percent in the dense tumor core. That’s a three-fold difference, suggesting this mechanism plays a role in cancer spread. Cells at the invasive edge of tumors likely face more physical squeezing as they push into surrounding tissues, making NAMs especially useful there.
The Cellular Scaffold Behind the Halo
NAM formation isn’t random. It depends on a supporting structure inside the cell:
- Actin filaments, the same protein fibers that power muscle contraction, wrap around the nucleus and trap mitochondria in place.
- The endoplasmic reticulum (ER) forms a mesh-like network around the nucleus, further helping to hold mitochondria close.
When researchers treated cells with latrunculin A, a drug that breaks down actin filaments, NAM formation collapsed and ATP delivery fell back to normal levels. This confirmed that the actin-ER scaffold is crucial for building and maintaining the mitochondrial halo.
Why This Matters for Cancer Therapy
Cancer treatment often struggles with the problem of metastasis — the spread of cancer cells to new parts of the body. For cells to metastasize, they must survive intense mechanical challenges: crawling through dense tumor tissue, slipping into narrow blood vessels, and withstanding pressure in the bloodstream.
The discovery of NAMs suggests that cancer cells survive these challenges by harnessing ATP surges. If scientists can find drugs that specifically disrupt NAM formation, they might be able to weaken cancer cells at their most vulnerable stage — without damaging healthy tissues.
For example, targeting the actin-ER scaffold could potentially block NAMs, cutting off emergency energy supplies. Unlike general mitochondrial poisons, this strategy might avoid harming normal cells that aren’t under the same stress.
A Universal Cellular Response?
While this study focused on cancer cells, the researchers believe NAMs may be a universal phenomenon in biology. After all, many types of cells experience physical squeezing:
- Immune cells squeeze through lymph nodes and tissues while hunting infections.
- Neurons stretch and extend branches during brain development.
- Embryonic cells face constant physical stress during morphogenesis.
In all these cases, a rapid nuclear ATP boost may be essential to safeguard DNA and maintain healthy cell function.
Rethinking the Role of Mitochondria
Traditionally, mitochondria have been seen as static batteries sitting in the cytoplasm, quietly producing energy. This discovery paints a different picture.
Mitochondria look more like mobile first responders, able to rush toward the nucleus in moments of crisis. Their ability to reposition themselves and deliver energy exactly where it’s needed could represent a broader principle in cell biology — one we are only beginning to understand.
The Bigger Picture: What This Teaches Us About Cells
This finding adds to a growing list of ways cells adapt to stress. For decades, biologists have studied chemical signals, genetic changes, and biochemical repair pathways. Now, physical forces and structural mechanics are being recognized as equally important.
The concept that cells can “feel” being squeezed and respond with an energy surge suggests that our understanding of biology has been missing a crucial layer. Mechanical stress is not just something cells endure — it actively shapes their survival strategies.
Related Insights into Mitochondria and Cancer
This isn’t the only recent finding about mitochondria’s surprising behavior in cancer:
- Some studies have shown that cancer cells can steal mitochondria from neighboring neurons to fuel metastasis.
- Mitochondria are known to play roles beyond energy production — including signaling, regulating cell death, and even influencing immune responses.
- The phenomenon of mitochondria clustering around specific regions (not just nuclei) has been observed in other contexts, like sperm motility and immune responses.
Together, these discoveries suggest mitochondria are much more versatile and adaptive than previously thought.
Implications for Future Research
This work opens new questions:
- Do all cancer types form NAMs, or only some?
- How common are NAMs in living human tumors beyond breast cancer?
- Can drugs be designed to target NAMs without harming normal cells?
- Do NAMs also form in non-cancer cells like immune cells under stress?
Answering these will not only deepen our understanding of cancer but also reveal more about how cells survive pressure in general.
Conclusion
The discovery of nucleus-associated mitochondria (NAMs) adds a completely new dimension to how we think about cancer survival and spread. By showing that cells under pressure can summon mitochondria to the nuclear surface for a rapid energy boost, researchers have uncovered a defensive system that could become a target for new cancer treatments.
Far from being static power plants, mitochondria are turning out to be agile, responsive organelles — ready to rush in when cells are literally pressed to the limit.