Scientists Uncover Why Alcohol Blocks Liver Regeneration, Even After You Quit

The liver is one of the most remarkable organs in the human body. It can regenerate itself after injury or even partial removal.
But a new study has uncovered why this natural healing power fails in people with alcohol-associated liver disease (ALD), even when they stop drinking. The research, led by scientists at the University of Illinois Urbana-Champaign in collaboration with Duke University and the Chan Zuckerberg Biohub Chicago, was published in Nature Communications on September 10, 2025.
The study shines light on how chronic alcohol consumption disrupts the liver’s ability to repair itself. It reveals that alcohol-induced inflammation interferes with RNA splicing, a critical step in protein production, leaving liver cells stuck in an in-between state where they are neither fully functional nor capable of regenerating. This discovery not only explains why advanced alcohol-related liver disease is so devastating but also points to new therapeutic targets that could one day restore liver regeneration.
The Big Picture: Alcohol and Liver Disease
Alcohol use is a leading cause of liver-related deaths worldwide, linked to an estimated 3 million deaths annually. Conditions such as alcohol-related hepatitis and alcohol-associated cirrhosis are often fatal, and once patients reach the stage of liver failure, the only effective treatment available is a transplant. Unfortunately, not all patients qualify, and donor organs are limited.

The liver’s natural ability to regenerate has long fascinated scientists and doctors. In healthy people, liver cells (hepatocytes) can reprogram themselves into a fetal-like progenitor state, multiply, and then transform back into fully mature, working liver cells. This process ensures recovery after injuries, infections, or even surgical removal of part of the liver. But in people with ALD, this regenerative power seems to fail. Until now, the reason for this failure wasn’t fully understood.
What the Researchers Found
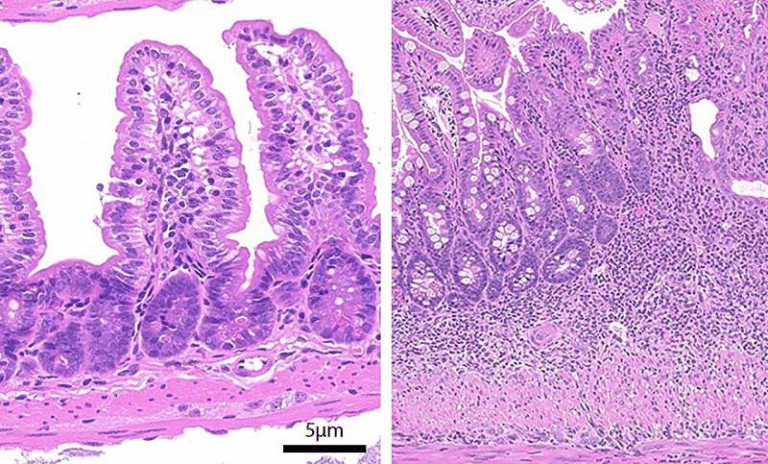
The team led by Auinash Kalsotra (University of Illinois) and Anna Mae Diehl (Duke University) compared healthy liver samples to those from patients with alcohol-associated hepatitis or alcohol-associated cirrhosis. Samples were provided through a collaboration with Johns Hopkins University Hospital and supported by the National Institute for Alcohol Abuse and Alcoholism (NIAAA).
Their detailed analysis showed something striking: in diseased livers, hepatocytes began to reprogram toward a regenerative state but became stuck halfway. Instead of successfully turning into proliferative progenitor cells and then back to functional hepatocytes, the cells ended up in a limbo state.
These trapped cells are not functional adult liver cells, but they’re also not effective progenitors. As a result, the remaining healthy cells in the liver are forced to work harder, creating stress that accelerates liver failure.
RNA Splicing: The Core Problem
To understand why these cells were stuck, the researchers turned to RNA sequencing. RNA splicing is a crucial cellular process where genetic instructions are cut and stitched together to form the final blueprint for protein production. If this process is disrupted, proteins may be made incorrectly, in the wrong place, or not at all.
The scientists discovered that RNA was being mis-spliced on a massive scale in ALD livers, across thousands of genes. This wasn’t just a small error—it was widespread dysfunction that directly affected protein function.
A key protein regulator stood out: ESRP2 (Epithelial Splicing Regulatory Protein 2). Normally, ESRP2 ensures proper splicing of RNA in liver cells. But in alcohol-damaged livers, ESRP2 levels were drastically reduced. Without enough ESRP2, the splicing machinery broke down, leaving essential proteins mislocalized and unable to carry out their roles in regeneration.
The Role of ESRP2 and Inflammation
To confirm ESRP2’s importance, the team looked at mice that lacked the ESRP2 gene. These animals showed similar liver damage and failed regeneration, mirroring what happens in humans with ALD.
But the researchers wanted to know why ESRP2 was missing in the first place. The culprit turned out to be inflammation. When the liver processes alcohol, it sustains cellular damage. In response, immune cells and liver-support cells flood the area, releasing large amounts of inflammatory and growth factors. These signals were found to suppress ESRP2 production and activity, triggering the mis-splicing problem.
To test whether this suppression could be reversed, the researchers treated liver cell cultures with a molecule that blocked the receptor of one inflammatory factor. Remarkably, ESRP2 levels bounced back and RNA splicing was restored. This finding highlights a potential pathway for therapeutic intervention.
Why This Matters
The study provides an entirely new way of looking at why livers fail to regenerate in alcohol-related disease. Instead of focusing only on cell death, scarring, or fibrosis, it shows that molecular miscommunication at the RNA level is a critical factor.
This opens up several exciting possibilities:
- Diagnostic markers: Specific mis-spliced RNAs could serve as biomarkers to predict which patients are at risk of regeneration failure.
- Therapeutics: Drugs that reduce liver inflammation or boost ESRP2 activity could help restore regenerative ability.
- Better understanding of recovery limits: Even if patients stop drinking, their livers may not bounce back unless these molecular defects are addressed.
Broader Context: Liver Regeneration Explained
To fully appreciate the significance of this research, it’s worth revisiting how the liver regenerates under normal conditions. Unlike most organs, the liver doesn’t rely on stem cells for repair. Instead, mature hepatocytes themselves can reprogram into a regenerative state.
- Step 1: Dedifferentiation – Cells shift into a more primitive, fetal-like state.
- Step 2: Proliferation – These progenitor-like cells multiply to replace lost tissue.
- Step 3: Redifferentiation – Cells mature again, resuming their specialized liver functions.
This process depends on precise gene expression programs and signaling pathways, including WNT and Hippo pathways. Any disruption can block regeneration. The discovery that RNA splicing defects are central to the problem in ALD adds an entirely new layer of understanding.
Alcohol and Its Lasting Impact
Even though many people assume that quitting alcohol will allow the liver to heal, this study shows it’s not always so simple. In advanced ALD, the damage to cellular machinery may persist even without ongoing alcohol use. This explains why some patients continue to worsen after they stop drinking.
It’s also important to note that alcohol isn’t the only cause of liver disease. Non-alcoholic fatty liver disease (NAFLD), viral hepatitis, and autoimmune conditions also impair regeneration, though often by different mechanisms. Research into RNA splicing may reveal common pathways across multiple liver diseases.
Where Does This Research Lead Next?
The study, while groundbreaking, is still at an early stage. Clinical translation will take time, but the roadmap is clearer:
- Test anti-inflammatory drugs that might rescue ESRP2 activity.
- Explore gene therapy approaches to restore ESRP2 directly.
- Develop biomarker tests for early detection of mis-splicing.
- Investigate whether other splicing regulators besides ESRP2 are involved.
Because the study used both human liver samples and mouse models, the evidence is strong, but more research is needed before new treatments become available.
Funding and Collaboration
The work was supported by the National Institutes of Health, the Chan Zuckerberg Biohub Chicago, the Duke Endowment, and the Muscular Dystrophy Association. Multiple NIH grants contributed, including R01-AA010154, R01-HL126845, R21-HD104039, 5R01-DK077794, 1R56-DK1343340, and R24 AA025017.
The collaboration included graduate students and researchers such as Ullas Chembazhi, Sushant Bangru, Diptatanu Das, and Subhashis Natua, among others. Their contributions spanned computational RNA sequencing, molecular biology, and cellular experiments.
Final Thoughts
This study represents a major step forward in understanding why alcohol-associated liver disease is so deadly and hard to treat. By pinpointing RNA splicing defects and ESRP2 suppression as central culprits, scientists now have a clearer path toward interventions that could revive the liver’s regenerative powers.
For millions of people worldwide suffering from liver disease, this discovery provides not only answers but also a foundation for hope that future treatments may restore what alcohol has taken away.