Shelf-Stable mRNA Delivery System Targets Bladder Cancer Cells Without Triggering an Immune Response
Published, peer-reviewed research from Purdue University has unveiled a new virus-mimicking mRNA delivery system that could significantly improve how messenger RNA therapies are delivered to bladder cancer cells. The technology, which is patent-pending and described in the Proceedings of the National Academy of Sciences (PNAS), addresses several long-standing challenges associated with existing mRNA delivery platforms, including storage limitations, immune system activation, and targeting efficiency.
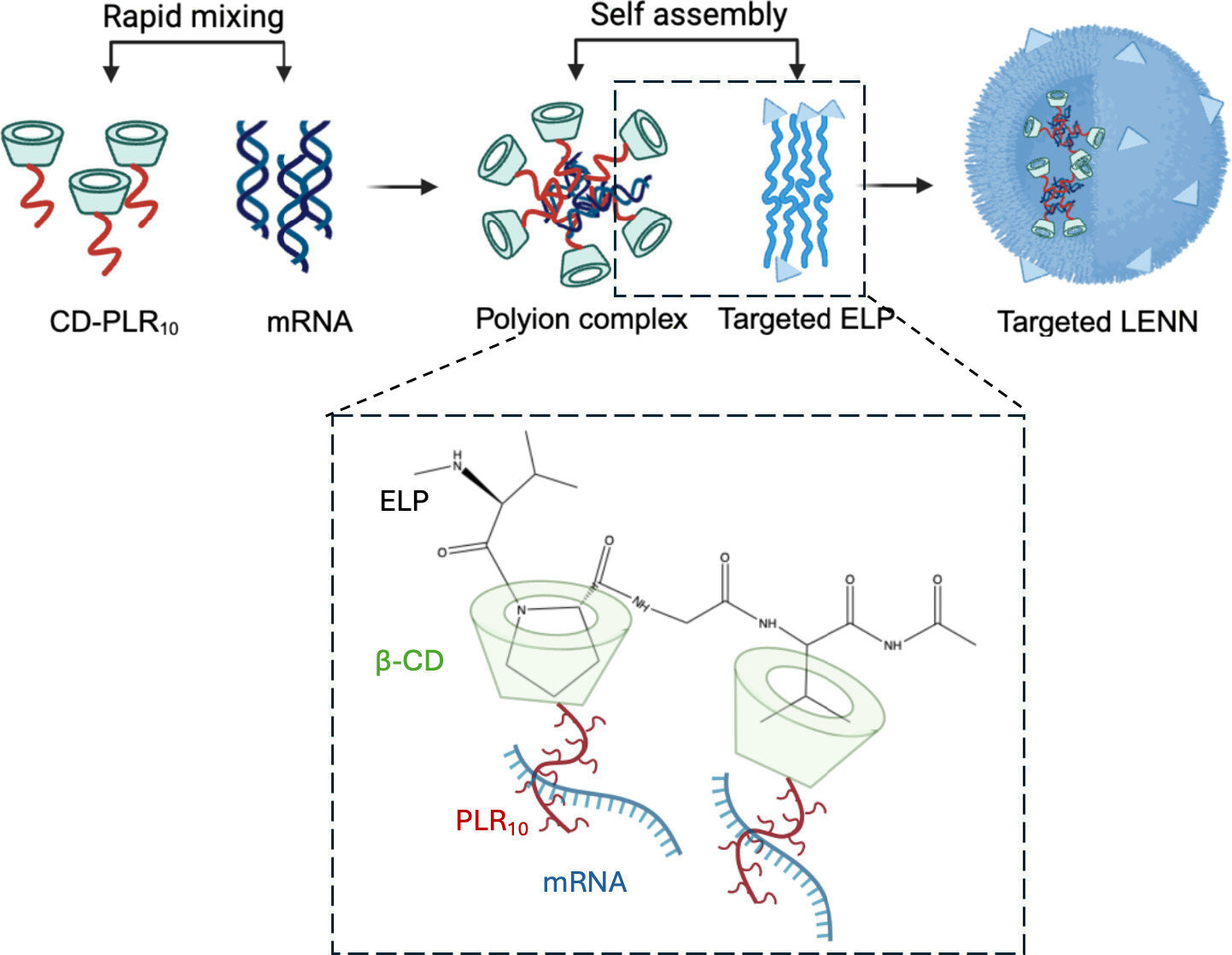
At the center of this research is a nanoparticle system known as LENN, short for layer-by-layer elastin-like polypeptide nucleic acid nanoparticle. The study demonstrates that LENN can deliver mRNA to bladder cancer cells while remaining shelf-stable, highly targetable, and biologically active after freeze-drying and rehydration—without provoking an immune response.
What Makes LENN Different From Existing mRNA Delivery Systems
Most mRNA therapies today rely on lipid nanoparticles. While effective, these systems come with serious drawbacks. They must be stored as liquids at ultra-low temperatures, often below –45°C, to preserve their activity. This creates logistical challenges, raises costs, and limits global accessibility.
The LENN system was designed to overcome these barriers. According to the research team, LENN can be lyophilized, or freeze-dried, into a powder that retains its structural integrity and biological function after rehydration. This alone represents a major step forward in making mRNA-based therapies more practical outside tightly controlled laboratory and hospital environments.
A Virus-Inspired Nanoparticle Design
LENN works by mimicking the multilayer structure of natural viruses, which have evolved to protect genetic material and deliver it efficiently into host cells. The nanoparticle consists of two distinct protective layers:
- An inner shell that electrostatically condenses and stabilizes the mRNA payload
- An outer shell made from elastin-like polypeptides that shields the mRNA from degradation and helps the particle evade immune detection
This layered architecture allows LENN to remain stable in circulation while delivering mRNA precisely where it is needed.
Targeting Bladder Cancer Cells With Precision
Bladder cancer presents unique challenges for drug delivery. Tumors in the bladder are difficult to target, and many delivery systems either fail to reach the cancer cells or enter them through unnatural pathways that can disrupt cellular behavior.
LENN avoids these issues by targeting a specific receptor already present on bladder cancer cells. Instead of forcing entry, the nanoparticle uses the cell’s natural endocytosis pathway, meaning it enters the cancer cell the same way naturally occurring molecules would. This approach helps preserve normal cellular processes while ensuring that the mRNA payload reaches its destination.
Once inside the cell, LENN releases the mRNA, allowing the cell’s machinery to produce the protein encoded by the mRNA, which is the fundamental goal of mRNA-based therapies.
No Detectable Immune Response
One of the most compelling findings in the study is that LENN does not trigger an immune response. Immune activation is a major limitation of many viral and non-viral delivery systems, as it can reduce therapeutic effectiveness and prevent repeat dosing.
The researchers confirmed that LENN neither alters the natural entry pathway of the tumor cells nor activates immune defenses. This opens the door to multiple dosing strategies, which are often essential in cancer treatment.
Freeze-Drying and Storage Stability
To test LENN’s stability, the researchers subjected the nanoparticles to a detailed freeze-drying process. Concentrated formulations were diluted, frozen at –20°C, further cooled to –80°C, and then lyophilized overnight. The resulting powders were stored at –20°C for three days.
After rehydration, the nanoparticles were evaluated for structural integrity, encapsulation efficiency, and biological performance. The results showed no meaningful difference between the freeze-dried samples and freshly prepared formulations. The system retained full functionality, making it a strong candidate for long-term storage and transport.
Green and Scalable Manufacturing
Another advantage of the LENN platform is its biomanufacturability. The system’s components are produced through biological expression, rather than complex chemical synthesis. This enables a more environmentally friendly (“green”) manufacturing process and makes the technology easier to scale for broader use.
The researchers emphasize that manufacturability is often overlooked in early-stage therapeutic research, but it plays a critical role in whether a technology can realistically reach patients.
Who Led the Research
The study was led by David Thompson, a professor in the James Tarpo Jr. and Margaret Tarpo Department of Chemistry at Purdue University. He is also affiliated with the Purdue Institute for Cancer Research and the Purdue Institute for Drug Discovery.
The paper’s lead author is Saloni Darji, a commercialization postdoctoral research associate, who played a central role in studying LENN’s stability, cellular entry mechanisms, and therapeutic potential.
What Comes Next for the LENN Platform
The next phase of development will focus on scaling up the system to support further preclinical testing. Planned studies include efficacy and safety evaluations in mouse models of bladder cancer, conducted in collaboration with Bennett Elzey from the Department of Comparative Pathobiology and the Purdue Institute for Cancer Research.
These studies will help determine whether LENN can move beyond laboratory validation toward real-world therapeutic applications.
Why This Research Matters Beyond Bladder Cancer
While the study focuses on bladder cancer, the implications of the LENN platform extend far beyond a single disease. A shelf-stable, immune-silent, targeted mRNA delivery system could transform how mRNA therapies are developed for cancer, genetic disorders, and potentially infectious diseases.
The ability to store mRNA therapies as powders rather than fragile frozen liquids could dramatically improve global distribution, particularly in regions without access to ultra-cold storage infrastructure.
A Broader Look at mRNA Delivery Challenges
mRNA therapies have gained global attention in recent years, but delivery remains the biggest bottleneck. Naked mRNA is unstable and easily degraded, and delivery vehicles must balance protection, targeting, cellular uptake, and immune compatibility.
The LENN system offers a promising example of how biomimicry, careful molecular design, and manufacturability considerations can come together to solve multiple problems at once.
Research Reference
Optimizing mRNA delivery with targeted elastin-like polypeptide–based LENN formulations: Insights into the endocytosis mechanism
Proceedings of the National Academy of Sciences (2026)
https://doi.org/10.1073/pnas.2502486122