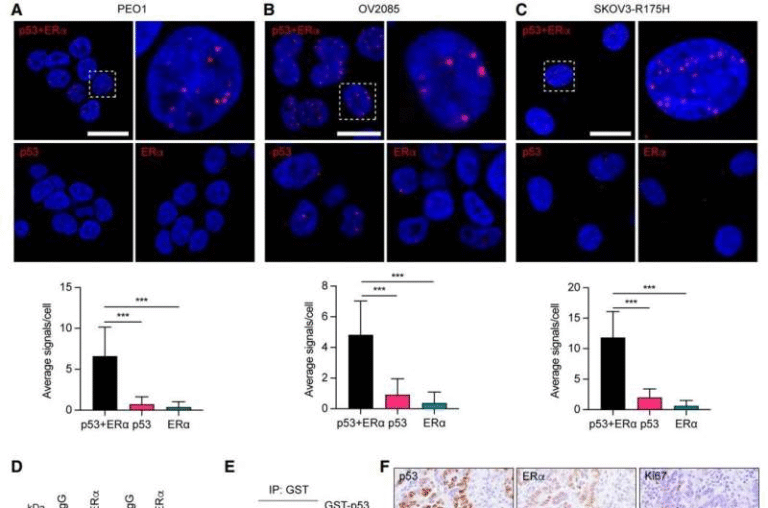
Special Breathing Tubes Do Not Improve Emergency Intubation Outcomes, Major Clinical Trial Finds
Emergency intubation is one of the most critical procedures in modern medicine, often performed on patients experiencing acute respiratory failure. Because these patients are already extremely vulnerable, even small improvements in equipment or technique could potentially save lives or prevent serious complications. That hope was at the center of a large new clinical trial examining whether modified endotracheal breathing tubes could outperform standard tubes in real-world emergency settings. The results, however, were clear and somewhat surprising: the specialized tubes did not lead to better patient outcomes.
The findings come from a randomized controlled phase 2 trial known as PreVent2, recently published in The Lancet Respiratory Medicine, one of the world’s leading journals in pulmonary and critical care research. Despite strong theoretical advantages and earlier supporting evidence, the trial showed that the modified tubes offered no meaningful benefit over standard breathing tubes when used during emergency intubation.
Why Modified Breathing Tubes Were Developed
Patients who require emergency intubation face several serious risks, one of the most concerning being ventilator-associated pneumonia (VAP). VAP is a lung infection that can develop after a patient is placed on mechanical ventilation. It is associated with longer ICU stays, higher mortality rates, impaired cognitive function, and reduced quality of life after recovery.
To reduce this risk, manufacturers developed a modified type of endotracheal tube called PU-EVAC. These tubes differ from standard versions in two key ways:
- They use polyurethane cuffs instead of traditional polyvinylchloride (PVC) cuffs, which are believed to form a tighter seal in the airway.
- They include subglottic secretion drainage, a built-in suction port designed to remove fluids that collect above the cuff and could otherwise leak into the lungs.
The idea was simple: reduce microaspiration, lower pneumonia risk, and improve overall outcomes for critically ill patients.
How the PreVent2 Trial Was Conducted
The PreVent2 trial was designed to test whether these theoretical benefits actually translate into real-world improvements. Researchers enrolled 1,068 adult patients who required emergency intubation due to acute respiratory failure.
Participants were randomly assigned to one of two groups:
- One group was intubated using the PU-EVAC endotracheal tube.
- The other group received a standard PVC endotracheal tube.
The study followed patients closely to track rates of suspected pneumonia, overall infections, throat injuries, and long-term recovery outcomes. This rigorous randomized design made PreVent2 one of the most comprehensive studies ever conducted on this topic.
What the Researchers Found
The results were striking in their consistency. Across all major outcomes, the specialized tubes failed to outperform standard tubes.
- Suspected pneumonia rates were nearly identical: 6% in the PU-EVAC group compared to 5% in the standard tube group.
- Overall infection rates were also similar, at 8% for PU-EVAC tubes versus 6% for standard tubes.
- There were no significant differences in throat injury risk between the two groups.
- Long-term recovery, including physical and cognitive outcomes, showed no meaningful improvement with the specialized tubes.
In short, the modified design did not deliver on its promise when used in emergency intubation scenarios.
Why These Findings Matter
Preventing ventilator-associated pneumonia has long been a top priority in intensive care medicine. Even modest reductions in infection rates can have a major impact on patient survival and healthcare costs. That is why earlier studies and meta-analyses had generated enthusiasm for PU-EVAC tubes, leading to their inclusion in some clinical guidelines.
However, the PreVent2 trial suggests that earlier evidence may not apply to emergency settings, where conditions are unpredictable and procedures must be performed rapidly. Emergency intubation differs significantly from planned ICU intubation, and those differences appear to matter.
Impact on Clinical Guidelines
Prior to this study, organizations such as the Centers for Disease Control and Prevention (CDC) and the Society for Healthcare Epidemiology of America had recommended the use of subglottic suction and polyurethane cuff tubes based on earlier data suggesting reduced pneumonia risk and shorter ICU stays.
In 2022, those recommendations were already downgraded due to growing uncertainty. The PreVent2 findings now provide strong clinical evidence supporting that decision. According to investigators involved in the trial, the results reinforce the idea that equipment changes alone are not enough to reduce complications in emergency intubation.
A Closer Look at Ventilator-Associated Pneumonia
Ventilator-associated pneumonia remains one of the most challenging complications in critical care. It develops when bacteria enter the lungs through the breathing tube, often via secretions that slip past the cuff. Despite advances in tube design, preventing VAP requires a multifaceted approach, including:
- Strict infection control practices
- Careful ventilator management
- Early patient mobilization when possible
- Judicious use of antibiotics
This trial highlights that no single device is likely to solve the problem on its own.
Emergency Intubation Is Still a High-Risk Procedure
One of the most important takeaways from the study is the reminder that patients requiring emergency intubation experience high morbidity and mortality, regardless of the type of tube used. Many survivors also face long-term cognitive challenges, emphasizing the need for continued research into better prevention and recovery strategies.
While the PU-EVAC tubes were safe and did not increase harm, their lack of added benefit suggests that resources may be better spent improving training, protocols, and post-intubation care, rather than relying on specialized equipment alone.
What This Means for the Future
The PreVent2 trial does not mark the end of innovation in airway management, but it does serve as a reality check. It underscores the importance of large, well-designed clinical trials before adopting new technologies into widespread practice. In emergency medicine especially, what works in theory or controlled settings may not hold up under real-world pressure.
For clinicians, the findings provide reassurance that standard endotracheal tubes remain appropriate for emergency intubation. For researchers, the study opens new questions about how best to reduce pneumonia risk and improve long-term outcomes for critically ill patients.
Research Paper Reference:
https://doi.org/10.1016/S2213-2600(25)00294-2