Stress May Disrupt Brain Blood Flow by Damaging Rare Neurons, New Research Finds

A new study from Penn State offers a detailed look into how chronic stress may interfere with the brain’s ability to regulate its own blood flow—and the key may lie in a very rare group of neurons that make up far less than 1% of the brain’s entire neural population. These neurons, known as type-one nNOS neurons, appear to play a surprisingly large role in maintaining healthy blood flow and coordinating electrical activity across the brain. When these neurons are lost—something that stress can trigger—the brain’s blood circulation and neural communication may suffer.
This research provides a clearer view of a long-observed connection: people with neurodegenerative conditions like Alzheimer’s disease and dementia often show reduced brain blood flow. Scientists have known that impaired circulation is a warning sign, but the reason has been unclear. The new findings suggest that stress-sensitive neurons may be part of that explanation.
Below is a full breakdown of the study and why it matters, followed by extra background information to help readers understand the neuroscience behind it.
What the Study Found
Researchers at Penn State focused on type-one nNOS neurons located in the somatosensory cortex, the region that processes sensations like touch and temperature. Although there are more than 20 different neuron types in any given brain region, this specific type plays a major role in regulating the spontaneous oscillation of blood vessels—tiny rhythmic expansions and contractions of arteries, veins, and capillaries that occur every few seconds. These oscillations help move oxygen and nutrients around the brain.
The study found that when these rare neurons were eliminated in mice, there was a noticeable drop in blood flow, a weakened amplitude of vascular oscillations, and a measurable reduction in neural electrical activity. In other words, removing a tiny fraction of neurons had a large and measurable impact on how the brain functioned.
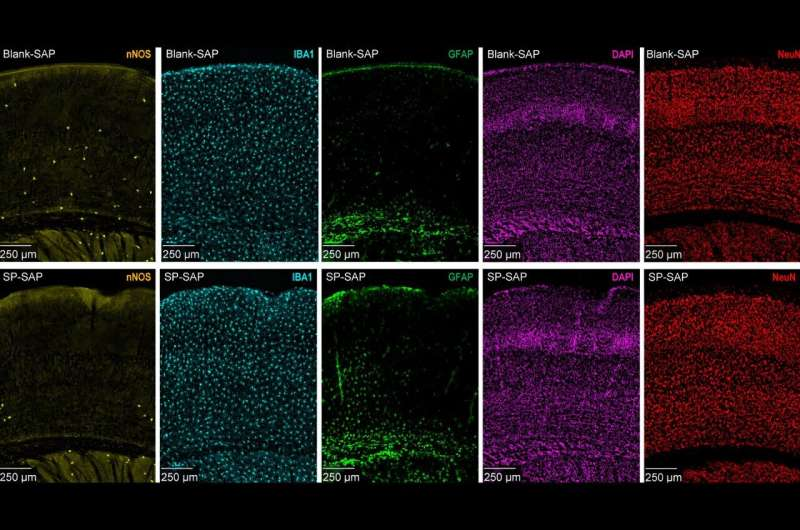
The neurons were selectively destroyed using an injection method designed to target them without damaging surrounding cells. The injected mixture contained saporin, a toxic protein that kills neurons, and a specially designed peptide that binds only to genetic markers found on type-one nNOS neurons. This approach allowed the researchers to remove these cells in a precise and controlled way.
After the injection, researchers measured brain changes using high-resolution imaging, electrodes, and behavioral tests. They monitored factors such as eye dilation, whisker movement, brain blood vessel oscillations at the micrometer scale, and electrical signals inside the brain. One standout observation is that disruptions in blood flow and neural activity were even stronger during sleep, suggesting these neurons may be especially important for nighttime brain processes.
The findings support a growing idea: long-term stress may gradually kill off these delicate neurons. Since they are essential for keeping blood flowing properly, losing them could reduce the brain’s ability to function at full capacity—and potentially increase vulnerability to neurodegenerative disease over time.
Why These Neurons Matter
Type-one nNOS neurons are not just rare—they are also extremely sensitive to stress. Previous research has shown that high levels of psychological stress can kill these cells more easily than others. This is important because nitric oxide, which these neurons help produce, is a key regulator of vasodilation, the widening of blood vessels.
Losing these neurons therefore means losing part of the brain’s internal plumbing system.
The study suggests that chronic stress may be an underestimated environmental factor in weakening the brain’s vascular system. While aging has long been recognized as a contributor to reduced blood flow, stress may silently be doing similar damage behind the scenes.
The research team notes that this work doesn’t yet prove a direct link to conditions like Alzheimer’s disease, but it lays the groundwork for future studies. Understanding how these neurons interact with known genetic risk factors could help explain why some people develop neurodegenerative diseases faster or more severely than others.
How the Researchers Carried Out the Experiment
To better understand how the brain behaves when these neurons are gone, the researchers used a carefully developed procedure:
- Targeted Neuron Elimination:
They used saporin attached to a peptide that recognizes and binds to markers found only on type-one nNOS neurons. This allowed the toxin to reach the intended cells without harming others. - Recording Blood Flow and Neural Activity:
After the neurons were destroyed, the scientists tracked changes in brain function using advanced optical imaging and electrodes. They recorded tiny oscillations in blood vessels—about 100 times smaller than the width of a human hair—as well as the electrical activity of surrounding neurons. - Measuring Behavioral Changes:
The researchers also monitored physical behavior such as eye dilation and whisker movement to understand how the neural changes translated into outward signs. - Comparing Awake and Sleeping States:
Notably, reductions in neural signaling and blood flow were even greater during sleep, implying these neurons help the brain transition into and maintain restorative sleep states.
This was the first time this neuron-targeting technique was used in this way, marking an important milestone in the study of neurovascular interactions.
What This Could Mean for Brain Health
The findings point to several important implications:
- Chronic stress may harm brain circulation.
If stress kills type-one nNOS neurons, the brain becomes less able to regulate blood flow, which may contribute to long-term cognitive decline. - Reduced blood flow could be a pathway to neurodegenerative disease.
Poor vascular function is already known to show up early in Alzheimer’s and dementia patients. - Sleep may be especially vulnerable to neuron loss.
Since blood flow disruptions were worse during sleep, this research adds to growing evidence that sleep and brain cleaning processes depend on proper blood circulation. - Future therapies might aim to protect or replace these neurons.
Although the research is still early, understanding the role of these neurons opens the door to treatments that preserve brain vasculature.
Additional Background: Understanding Brain Blood Flow
To give you a bit more context, the brain uses about 20% of the body’s oxygen, even though it makes up only a small portion of body weight. Because of this, it relies heavily on precise and constant blood flow.
Here are some additional concepts relevant to this study:
Neurovascular Coupling
This refers to how neurons signal nearby blood vessels to expand when they need more oxygen and nutrients. When this process goes wrong, brain performance suffers.
Vasomotion
These are the natural rhythmic pulses in blood vessels. They help distribute nutrients and remove waste. The study found that eliminating type-one nNOS neurons weakened these oscillations significantly.
Nitric Oxide’s Role
Nitric oxide acts like a chemical messenger that tells blood vessels when to relax and expand. Neurons that produce this molecule are essential for keeping the brain’s circulation stable.
Understanding these mechanisms helps make sense of why such a tiny group of neurons is so crucial—and why stress can have such an outsized impact.
Research Paper
Type-I nNOS neurons orchestrate cortical neural activity and vasomotion
https://doi.org/10.7554/elife.105649.3