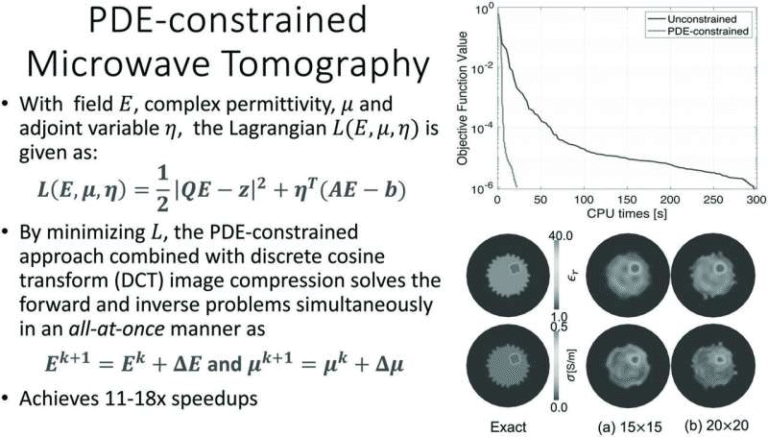
Targeting Epigenetic Modifiers and Splicing Regulators Together Could Open New Treatment Paths for Acute Myeloid Leukemia
Acute myeloid leukemia (AML) is a complex blood cancer, and treating it effectively depends on understanding what goes wrong inside leukemia cells at a molecular level. A newly published study offers an important piece of that puzzle by showing how two common genetic mutations—IDH2 and SRSF2—can work together to disrupt normal cell behavior. The findings suggest that tackling both mutations at once may point toward new combination treatment strategies for certain AML patients.
The research, carried out by scientists at the Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine, was published in Science Advances in early 2026. Rather than looking at genetic mutations in isolation, the team focused on how these mutations interact, revealing a cooperative mechanism that reshapes how blood cells develop and function.
Understanding AML at the Molecular Level
AML arises when immature blood cells fail to develop properly and begin to multiply uncontrollably. At the heart of this process are changes in how genes are regulated and expressed. While mutations in genes like IDH2 and SRSF2 have been studied individually for years, patients often carry multiple mutations, and those combinations can create unique disease behaviors.
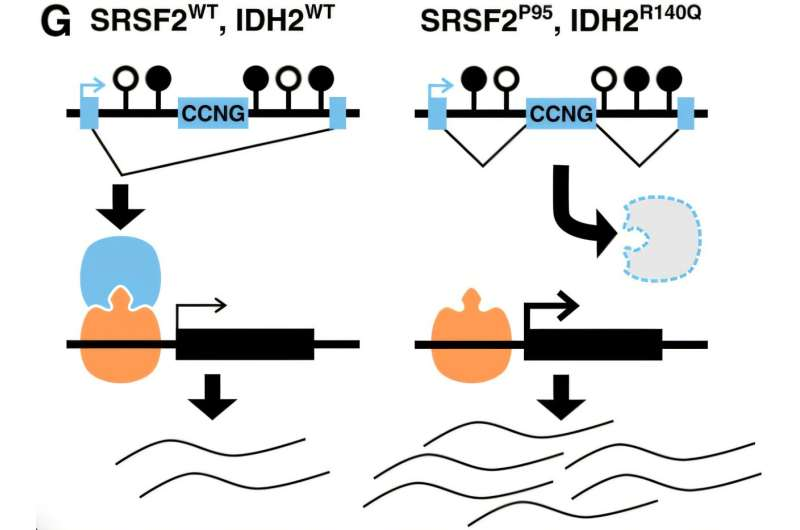
This study zeroes in on that idea. The researchers found that when IDH2 and SRSF2 mutations occur together, they produce effects that are more severe—and more specific—than either mutation alone. The result is widespread disruption of RNA splicing, a process that is essential for producing functional proteins.
Why RNA Splicing Matters So Much
RNA splicing is the step where raw genetic messages are edited before being turned into proteins. Segments of RNA are cut out or stitched together so that the final message makes sense. If splicing goes wrong, cells can end up producing faulty or harmful proteins.
The SRSF2 gene plays a direct role in this editing process, helping decide which RNA segments are kept and which are removed. Mutations in SRSF2 are already known to cause splicing errors. What makes this study stand out is how it shows that IDH2 mutations amplify this problem.
IDH2 doesn’t control splicing directly. Instead, it alters the epigenetic landscape of the cell—specifically, chemical tags on DNA known as methylation marks. These tags help guide how genes are read and processed. When IDH2 is mutated, those chemical signals change, creating an environment where splicing decisions become increasingly error-prone.
A Harmful Partnership Between IDH2 and SRSF2
When both mutations are present, the researchers observed a dramatic increase in splicing errors compared to cells with only one mutation. These errors were not random. They clustered near regions of DNA where methylation patterns had been altered, suggesting a direct link between epigenetic changes and faulty RNA splicing.
The genes most affected by this mis-splicing tended to be long and structurally complex, making them especially vulnerable to disruption. Many of these genes encode transcriptional regulators—proteins that act as master switches controlling cell identity and development. When those regulators are mis-spliced, the downstream effects can fundamentally alter what a cell becomes and how it behaves.
In AML, this means blood cells can lose their normal identity and remain stuck in an immature, leukemia-driving state.
Using Advanced Tools to Map the Damage
To uncover these mechanisms, the research team analyzed patient samples and laboratory models using high-resolution RNA sequencing and DNA methylation mapping. They didn’t stop there. The scientists also applied artificial intelligence models to the data.
One striking result was that DNA methylation patterns alone could be used to predict splicing outcomes with surprising accuracy. This finding strengthens the idea that epigenetic changes are not just background noise but are actively shaping how RNA is processed in AML cells with these mutations.
This predictive link opens the door to more precise ways of identifying which patients might benefit from therapies that target epigenetic regulation.
Implications for Treatment Strategies
The study’s most exciting implication lies in therapy. IDH2 and SRSF2 affect two different but interconnected systems: epigenetic regulation and RNA splicing. By showing that these systems reinforce each other’s mistakes, the research suggests that treating only one may not be enough.
In laboratory experiments, AML cells carrying both mutations showed increased sensitivity to romidepsin, a drug that inhibits chromatin-modifying enzymes. Romidepsin is already approved for certain lymphomas, and while it is not currently a standard treatment for AML, this sensitivity hints at a potential vulnerability in double-mutant leukemia cells.
The idea is not necessarily to replace existing treatments but to combine epigenetic therapies with approaches that influence splicing regulation, potentially making leukemia cells more susceptible to treatment.
Why This Matters for Precision Medicine
AML is increasingly understood as a collection of biologically distinct subtypes rather than a single disease. This study reinforces that view by demonstrating that specific mutation combinations create specific molecular problems.
For patients with both IDH2 and SRSF2 mutations, therapies designed with this interaction in mind could offer better outcomes than one-size-fits-all approaches. The detailed mechanistic map provided by this research gives clinicians and researchers a clearer roadmap for designing future clinical trials.
A Broader Look at Epigenetics and Splicing in Cancer
Beyond AML, this work adds to a growing body of evidence showing that see epigenetics and RNA processing as deeply interconnected. Epigenetic changes influence not just whether a gene is turned on or off, but also how its message is assembled. In cancers driven by complex mutation patterns, this layered regulation may be a common theme.
As research continues, similar cooperative mechanisms may be uncovered in other blood cancers and solid tumors, potentially widening the impact of these findings well beyond AML.
Looking Ahead
This study doesn’t offer an immediate cure, but it delivers something just as important: clarity. By showing exactly how IDH2 and SRSF2 mutations cooperate to derail normal cell development, it provides a strong scientific foundation for future therapies.
As researchers continue to explore combination treatments and refine epigenetic drugs, patients with these mutations may eventually benefit from therapies that restore proper gene regulation—or at least silence the most damaging errors. Understanding how cells lose their script is the first step toward helping them find it again.
Research paper:
https://www.science.org/doi/10.1126/sciadv.adu8292