Unexpected Finding Could Offer New Treatment Targets for Meth Addiction

University of Florida neuroscientists have uncovered a surprising biological mechanism that could open the door to entirely new ways of treating methamphetamine addiction, a condition that currently has no FDA-approved medications. The discovery links the brain’s immune signaling system directly to the dopamine-driven reward pathways that fuel addiction, offering a fresh angle on a long-standing public health challenge.
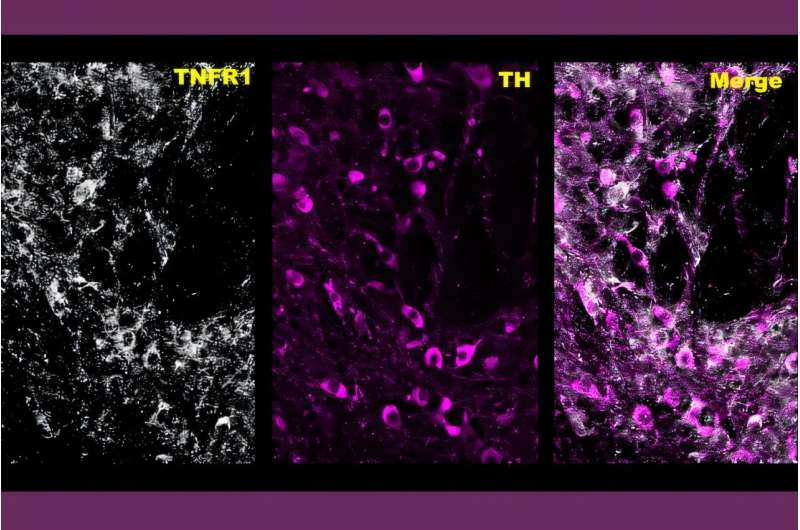
The research, led by Habibeh Khoshbouei, a professor of neuroscience and psychiatry at the University of Florida’s McKnight Brain Institute, focuses on how methamphetamine affects neuroinflammation and dopamine signaling in the brain. While meth has long been known to flood the brain with dopamine, this study reveals that the drug also activates an inflammatory molecule that plays a much more active role in reinforcing addictive behavior than previously thought.
Meth Addiction and the Dopamine Problem
Methamphetamine is a highly addictive psychostimulant that produces intense feelings of pleasure and euphoria. It does this primarily by boosting levels of dopamine, a neurotransmitter involved in motivation, reward, and learning. Repeated exposure to meth can severely disrupt normal dopamine signaling, driving cravings, compulsive drug use, and relapse.
Unlike addiction to alcohol or opioids, which can be treated with medications such as naltrexone or buprenorphine, meth addiction has no approved pharmaceutical treatment. This gap has made it especially difficult for clinicians to help people break the cycle of dependence.
Meth also causes a range of damaging physical effects, including chronic inflammation, impaired wound healing, and dental decay commonly referred to as meth mouth. These inflammatory effects were once thought to be secondary consequences of drug use. The new research suggests they may be much more central to addiction itself.
A Closer Look at Brain Inflammation
The study, published in Science Signaling, examined mouse brain tissue to explore how meth influences inflammatory signaling in dopamine-producing neurons. The researchers focused on tumor necrosis factor-alpha (TNF-α), a powerful immune protein known for regulating both acute and chronic inflammation throughout the body.
What they found was unexpected. Methamphetamine did not just increase dopamine release. It also triggered a spike in TNF-α signaling within the brain’s dopamine system. Even more surprising, TNF-α was not simply responding to damage or stress—it actively enhanced dopamine neuron activity.
Using electrophysiological recordings, the team observed that TNF-α increased the firing rate of dopaminergic neurons in the midbrain. In other words, an immune molecule typically associated with inflammation was directly amplifying the same neural activity that makes meth so addictive.
Dopamine Transporters and Calcium Channels
The study also identified the molecular machinery behind this interaction. TNF-α was found to stimulate dopamine transporters, proteins that regulate dopamine levels by controlling how quickly it is taken back up into neurons. At the same time, TNF-α activated L-type calcium channels, allowing more calcium to flow into neurons and increasing their excitability.
Methamphetamine itself affects these same systems. By stimulating dopamine transporters and calcium channels, meth and TNF-α appear to work together in a feedback loop that strengthens dopamine signaling and reinforces addictive behavior.
Crucially, when researchers used chemical compounds to block either the dopamine transporter or TNF-α signaling, the effects of meth were significantly reduced. Blocking the TNF receptor also blocked the dopamine-boosting effects of TNF-α itself, showing that the two systems are tightly linked.
Why This Discovery Matters
This finding reshapes how scientists think about addiction. Traditionally, dopamine has been viewed as the main driver, while inflammation was seen as a downstream consequence. This study suggests that immune signaling is an active participant in shaping addictive neural circuits.
One of the most promising aspects of this discovery is that TNF-α inhibitors already exist. These drugs are widely used to treat autoimmune and inflammatory diseases such as Crohn’s disease, rheumatoid arthritis, and psoriasis. Because they are already approved by the U.S. Food and Drug Administration, they could potentially be repurposed for addiction research more quickly than entirely new drugs.
The researchers emphasize that this is still early-stage work. The study was conducted using preclinical models, and extensive testing would be needed before any TNF-targeting drug could be considered for human use in addiction treatment. Still, identifying a drug-accessible target is a major step forward.
Implications for Reducing Cravings and Relapse
Beyond reducing inflammation, targeting TNF-α could influence drug-seeking behavior itself. By dampening dopamine hyperactivity, immune-modulating therapies may help reduce cravings, compulsive use, and relapse risk. The researchers plan to explore how TNF-α signaling affects different behavioral aspects of addiction, including motivation and reinforcement.
This approach also raises broader questions about whether inflammation plays a similar role in other substance use disorders. If immune molecules can directly shape reward circuits, future addiction treatments may involve a combination of neurological and immunological strategies.
Understanding TNF-α Beyond Addiction
TNF-α is a cytokine, a type of signaling protein used by the immune system to coordinate responses to injury and infection. While essential for normal immune function, excessive or chronic TNF-α activity can damage tissues and disrupt normal cellular signaling. In the brain, TNF-α has been linked to neurodegenerative diseases, depression, and cognitive decline.
The idea that TNF-α can also enhance dopamine release challenges long-held assumptions about how immune molecules function in the nervous system. It highlights the growing recognition that the brain and immune system are deeply interconnected.
A New Direction for Addiction Research
Methamphetamine addiction continues to be a major global health issue, contributing to overdose deaths, mental health disorders, and long-term neurological damage. Discoveries like this one offer hope not by providing an immediate cure, but by expanding the scientific playbook for tackling addiction.
By showing that immune signaling molecules such as TNF-α can directly influence dopamine pathways, this research opens new avenues for understanding why meth is so addictive and how its grip might be loosened. It also underscores the importance of looking beyond traditional neurotransmitters when studying complex brain disorders.
As future studies explore how immune modulation affects behavior, cravings, and relapse, this unexpected link between inflammation and dopamine could reshape how addiction is treated in the years ahead.
Research paper: https://www.science.org/doi/10.1126/scisignal.ady8676