Cambridge Scientists Discover a Quantum Trick in Organic Molecules That Could Transform Solar Power
In a breakthrough blending quantum physics and organic chemistry, researchers at the University of Cambridge have discovered a completely new way to turn light into electricity — using a single type of organic molecule. This discovery challenges one of the core assumptions in solar technology and could eventually lead to lighter, cheaper, and simpler solar panels made entirely from one material.
The study, published in Nature Materials on September 30, 2025, describes a hidden quantum mechanism inside a specially designed organic semiconductor. For decades, scientists believed such behavior could only happen in inorganic metal oxides, not soft, carbon-based materials. But this new finding shows that organic semiconductors—materials similar to those used in flexible electronics and OLED displays—can achieve similar quantum effects.
The Star of the Discovery: P3TTM
At the heart of this research lies an unusual molecule called P3TTM, short for poly(3-triphenylmethyl-thiophene). It’s what’s known as a spin-radical organic semiconductor — meaning each molecule has one unpaired electron that gives it a distinctive magnetic and electronic personality.
Most organic materials are “closed-shell,” where electrons are paired and stable. In contrast, P3TTM is “open-shell,” hosting that lone unpaired electron. This feature allows the molecules to interact in a way that’s very different from typical semiconductors.
This work came from a collaboration between Professor Hugo Bronstein’s synthetic chemistry group in the Yusuf Hamied Department of Chemistry and Professor Sir Richard Friend’s physics group at the Cavendish Laboratory, both at Cambridge. Together, they designed and studied P3TTM molecules to understand how light interacts with these radicals.
How the Discovery Works
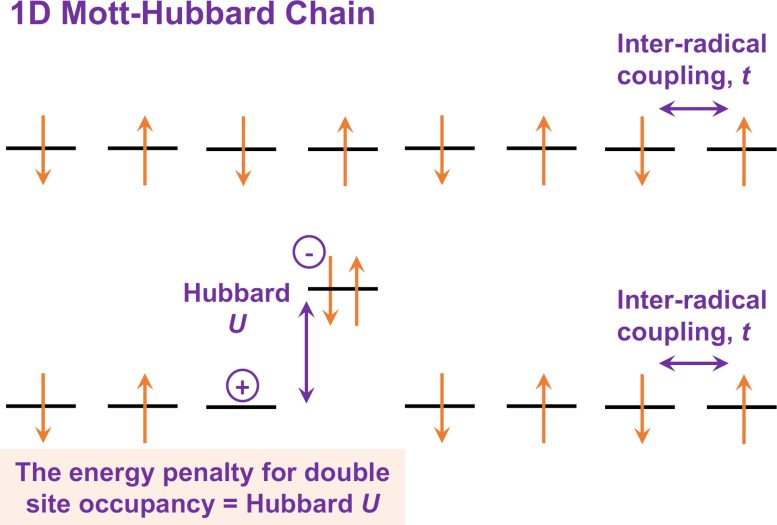
When the researchers shined light on thin films made from P3TTM, they noticed something remarkable: instead of acting like a typical organic semiconductor, the molecules behaved as if they were part of a Mott–Hubbard system — a kind of material described by quantum physics to explain how strongly interacting electrons behave.
In simple terms, when neighboring P3TTM molecules sit close together, the unpaired electrons on each one interact and align alternately, up and down, similar to how magnetic spins align in quantum materials known as Mott–Hubbard insulators.
Upon absorbing light, one of these electrons hops from its home molecule to a neighbor. This hop creates two regions: one positively charged and the other negatively charged. These separated charges can then be collected as electric current — the essence of solar power.
That’s a big deal because, in most organic solar cells, charge separation only happens at the interface between two different materials — one that donates electrons (the donor) and one that accepts them (the acceptor). The interface is crucial, but it limits efficiency. Here, charge separation happens within a single, uniform material.
This is why the researchers describe the phenomenon as “intrinsic intermolecular photoinduced charge separation.”
Close to Perfect Charge Conversion
When the team made a simple solar cell using only P3TTM, they observed something astounding: nearly every photon that hit the material was converted into an electrical charge. In scientific terms, the device achieved close-to-unity charge collection efficiency.
That means almost no light was wasted in the conversion process — an impressive feat for any solar material, let alone a new organic one.
In traditional organic photovoltaics (OPVs), much of the absorbed light energy gets trapped as excitons — bound electron-hole pairs that are hard to separate. This is why most OPVs rely on complex donor-acceptor blends. P3TTM doesn’t need that. Instead, because of the molecule’s radical nature and its quantum-level interactions, the electrons can separate and move freely, even without a second material.
This discovery shows that organic molecules can generate charges all by themselves, which was previously thought to be impossible.
A Quantum Echo from the Past
The Cambridge team also points out the historical importance of this finding. The underlying physics is linked to the work of Sir Nevill Mott, a British physicist whose theories on electron interactions helped shape modern semiconductor science.
Professor Sir Richard Friend, one of the senior authors, worked early in his career with Mott. The discovery, published exactly 120 years after Mott’s birth, serves as a fitting tribute to his pioneering insights into electron behavior in solids.
It’s a rare moment when a century-old theory comes alive again in a totally new kind of material — one that could now shape the future of solar technology.
The Science Behind the Efficiency
The behavior of these molecules depends on a delicate energy balance described by Mott–Hubbard physics.
There’s an energy barrier called Hubbard U — the electrostatic cost of putting two electrons on the same molecule. In P3TTM, the researchers designed the molecular structure so that the energy of an absorbed photon makes it energetically favorable (“downhill”) for an electron to jump from one molecule to its neighbor.
In simpler terms: once the molecule absorbs light, it naturally prefers to split the charge rather than keep the energy bound up.
Dr. Petri Murto, also from Cambridge’s Department of Chemistry, fine-tuned the P3TTM molecular design to control how closely molecules pack together and how their energy levels align. This precise tuning made the charge-separation process efficient and stable.
The team fabricated thin films of P3TTM and observed red luminescence — evidence that the excited radical states were interacting and releasing energy in a controlled way. These films glowed brightly when excited, similar to how organic LEDs (OLEDs) work, but with an added twist: they were also generating electricity.
Building a Solar Cell from a Single Material
One of the biggest challenges in organic solar technology has been complexity. To make solar cells efficient, scientists typically need to combine two materials with perfectly matched energy levels. This requires nanoscale mixing and precise layering.
With this new mechanism, however, a solar cell could theoretically be built using just one organic compound. That means fewer manufacturing steps, lighter weight, and lower costs.
Organic materials like P3TTM are also flexible and can be processed at low temperatures, making them ideal for wearable electronics, building-integrated solar panels, or even solar textiles.
While more work remains to test durability and long-term efficiency, the discovery opens the door to a new generation of organic solar cells that are simpler and more sustainable.
What Makes Mott–Hubbard Physics So Important
The Mott–Hubbard model is a cornerstone of condensed matter physics, describing how electrons behave in materials where their interactions dominate over simple band behavior.
In most conductors, electrons move freely in a delocalized “sea.” But in Mott–Hubbard systems, electrons strongly repel each other, creating insulating or magnetic states even when conventional band theory would predict metallic behavior.
Seeing such physics appear in organic molecules is extraordinary. Traditionally, Mott–Hubbard effects were only found in inorganic crystals such as transition metal oxides. By showing these effects in carbon-based radicals, the Cambridge team bridged two worlds that rarely meet: molecular chemistry and quantum correlated systems.
This opens up possibilities not just for solar energy, but for quantum electronics, spintronics, and magnetic semiconductors made from organic materials.
Challenges Ahead
Despite the excitement, there are still some challenges before this discovery can power your rooftop panels:
- Stability – Organic radicals can be chemically reactive and degrade over time. Making these materials robust in sunlight and air will be crucial.
- Scalability – The thin films used in the experiments were created in controlled lab conditions. Scaling that to large-area solar panels will take engineering advances.
- Full Device Efficiency – While charge collection is nearly perfect, full power conversion efficiency (PCE) also depends on voltage, current, and stability. Real-world performance tests are the next step.
- Material Design – The exact balance of energy levels that makes P3TTM work is delicate. Researchers will need to see whether other molecules can replicate or improve upon this behavior.
Still, the implications are profound. The idea that a single organic molecule can act as both light absorber and charge generator could rewrite the playbook for solar cell design.
A New Chapter for Organic Solar Energy
For decades, scientists have dreamed of building simple, efficient, and flexible solar cells out of organic materials. The promise was always there: cheap production, tunable chemistry, and mechanical flexibility. But organic photovoltaics struggled because of low efficiency and stability.
This new discovery might change that. By proving that open-shell organic radicals like P3TTM can separate charge intrinsically, Cambridge researchers have revealed a new pathway that sidesteps many of the limitations of current designs.
It’s still early, but the potential is massive. If scientists can build on this discovery, we could soon see fully organic solar panels that are not just lightweight and efficient, but also environmentally friendly and cost-effective.