New Research Reinvents MXene Synthesis and Slashes Costs by Orders of Magnitude
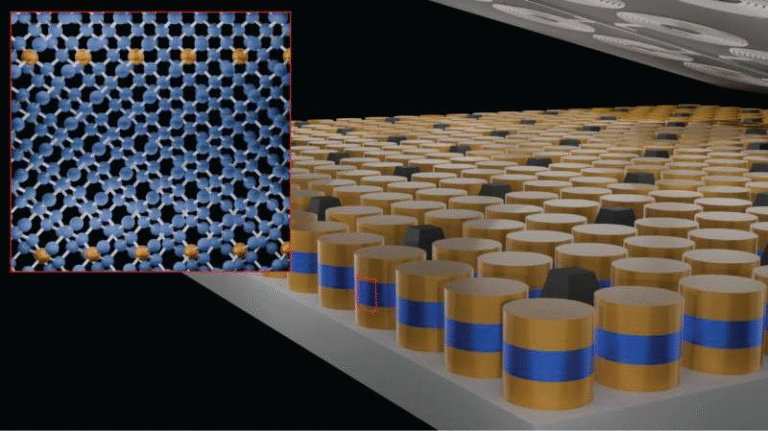
MXenes are a relatively young but extremely promising class of two-dimensional materials, first identified only about 14 years ago, and they have been drawing growing attention across physics, chemistry, and engineering. These materials are made of atomically thin layers of transition metals combined with carbon or nitrogen, and they stand out because of their exceptional electrical conductivity, chemical tunability, and ability to host ions between their layers. As a result, MXenes are being explored for energy storage, industrial catalysis, electromagnetic interference shielding, ultrastrong lightweight composites, optoelectronics, and even conductive inks.
Despite all this potential, MXenes have faced one stubborn problem from the beginning: they are hard, expensive, and unsafe to make at scale. That challenge may now be close to being solved.
A new study led by researchers from the University of Chicago, University of Illinois Chicago, and Vanderbilt University reports a fundamentally different way to synthesize MXenes—one that dramatically cuts costs, improves purity, and avoids the dangerous chemicals traditionally used in MXene production. The research was recently published in Nature Synthesis and represents a major shift in how these materials can be manufactured.
Why Traditional MXene Manufacturing Has Been a Problem
Until now, MXenes have almost exclusively been produced using what is known as a top-down synthesis approach. In this method, scientists start with layered bulk materials called MAX phases and chemically “etch” away certain atomic layers to leave behind ultrathin MXene sheets.
This process has several serious drawbacks. It typically involves multiple high-temperature steps that can take days to complete. Even more problematic, it often relies on highly corrosive and toxic chemicals, including hydrofluoric acid, molten salts, or similar caustic reagents. These chemicals pose obvious safety risks, generate large amounts of hazardous waste, and significantly drive up production costs.
While this approach was acceptable for early laboratory research, it became a major bottleneck for scaling MXenes toward real-world applications. The cost, complexity, and environmental concerns made it difficult to imagine widespread industrial use.
A Bottom-Up Approach That Changes the Equation
The new research flips the entire process on its head. Instead of carving MXenes out of larger materials, the team developed a bottom-up synthesis method that builds MXenes atom by atom, much more like how crystals are grown.
Using chemical vapor deposition (CVD), the researchers directly formed two-dimensional MXene layers from carefully chosen molecular precursors. This allowed them to precisely control the composition and structure of the material from the start, rather than relying on aggressive chemical removal after the fact.
According to the researchers, MXenes produced using this new technique are at least two orders of magnitude cheaper than those made with conventional methods. Just as important, the process is far safer, produces far less waste, and is much more compatible with industrial-scale manufacturing.
Safer Chemicals, Higher Yields, Better Control
One of the most striking improvements in the new method is the choice of precursor chemicals. Earlier experiments relied on extremely reactive compounds such as titanium tetrachloride, which can be so aggressive that it damages laboratory equipment during handling.
In contrast, the new work uses molecular organohalides, including tetrachloroethylene, a stable and inexpensive compound commonly used in industrial processes such as coffee decaffeination. This change alone represents a major step forward in safety and cost reduction.
The improvements extend beyond chemistry. Earlier attempts at bottom-up MXene synthesis struggled to achieve high purity, topping out at around 60 weight percent MXene content. In this latest study, the researchers achieved around 90 weight percent, a dramatic leap that signals the process is becoming both reliable and reproducible.
Inspiration From a Forgotten Paper
Interestingly, the roots of this breakthrough trace back nearly four decades. The researchers drew inspiration from a largely overlooked 1986 paper by chemist John Corbett at Iowa State University. That work described a way to synthesize layered zirconium chloride carbide—materials that share important structural similarities with MXenes.
By revisiting and adapting this older chemistry using modern tools and computational insights, the team was able to unlock a new reaction pathway that had gone largely unnoticed. It is a powerful reminder that fundamental, curiosity-driven research can have long-lasting and unexpected impacts.
What Makes MXenes So Special?
To understand why this breakthrough matters, it helps to look at what makes MXenes unique in the first place.
MXenes consist of conductive two-dimensional layers that can store ions between them. This property makes them especially attractive for batteries and supercapacitors, where fast ion transport and high electrical conductivity are critical.
Another key feature is their tunable surface chemistry. Scientists can modify the surface groups on MXenes to control which ions they interact with, how easily those ions move, and how stable the material remains during repeated charging and discharging cycles.
Because of this versatility, MXenes are being investigated for:
- Energy storage systems
- Industrial and electrochemical catalysts
- Electromagnetic shielding materials
- Flexible electronics and sensors
- Conductive inks for printable electronics
The biggest obstacle has always been making enough of them safely and cheaply. This new synthesis method directly addresses that challenge.
The Role of the M-STAR Research Consortium
The work was carried out under the umbrella of the NSF Center for Chemical Innovation on MXenes Synthesis, Tunability and Reactivity (M-STAR). This collaborative framework brings together chemists, materials scientists, physicists, and chemical engineers to tackle MXene-related challenges from multiple angles.
By combining traditional inorganic chemistry, nanoscale synthesis, catalysis, and computational modeling, the team was able to refine both the chemistry and the underlying theory behind the new process. This interdisciplinary approach is likely to play a major role in pushing MXenes from the lab into practical technologies.
What This Means Going Forward
This research does more than just improve an existing process—it redefines how MXenes can be made. By eliminating dangerous chemicals, slashing costs, improving yields, and enabling better control over material composition, the new bottom-up synthesis method opens the door to large-scale MXene production.
That, in turn, could accelerate progress in energy storage, electronics, catalysis, and other cutting-edge technologies that rely on advanced materials. Just as importantly, it highlights the value of revisiting older scientific ideas with fresh perspectives and modern tools.
MXenes may still be young materials, but with breakthroughs like this, they are rapidly moving from experimental curiosities to serious industrial contenders.
Research paper:
https://doi.org/10.1038/s44160-025-00946-w