Physicists Develop a Molecule-Based Method to Peer Inside an Atom’s Nucleus
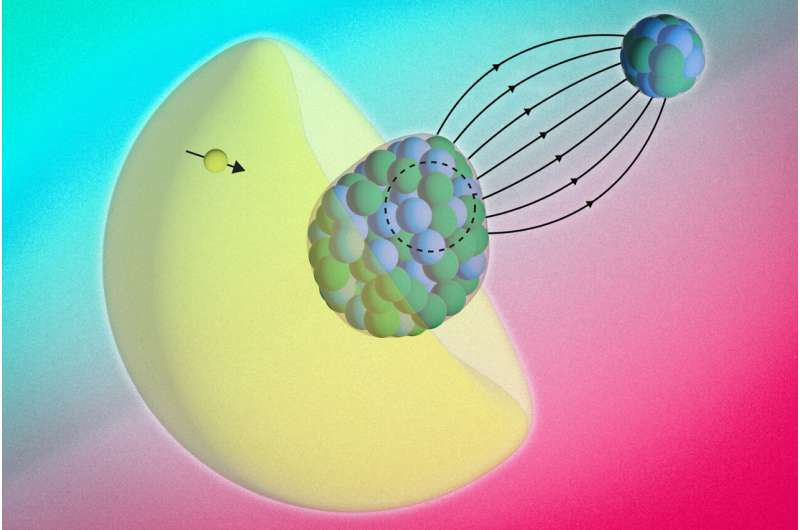
Physicists at MIT have achieved something remarkable — they’ve figured out a new way to look inside an atom’s nucleus without needing gigantic particle accelerators. Their innovative approach uses molecules themselves as miniature laboratories, letting electrons act as messengers that carry subtle information from the very heart of the atom.
The study, recently published in Science, demonstrates how a molecule called radium monofluoride (RaF) can reveal details about the nucleus of a radium atom. Instead of firing high-energy beams at atomic nuclei, as traditional experiments do, the team used the natural environment inside the molecule to probe the nucleus from within.
Let’s unpack exactly how this works, why it’s so groundbreaking, and what it could mean for the biggest unanswered questions in physics — including why the universe is made mostly of matter instead of antimatter.
How the Experiment Works
The experiment revolved around the molecule radium monofluoride, which is made by pairing a radium atom (Ra) with a fluoride atom (F). This combination forms a molecule where the radium atom sits inside an electric field that is far stronger than anything scientists can generate in a lab. That strong internal field compresses the electrons around the radium atom, dramatically increasing the chance that these electrons will briefly penetrate the nucleus itself.
When this happens, the electrons can interact directly with the protons and neutrons inside the nucleus — the building blocks of atomic matter. These fleeting interactions leave measurable fingerprints in the form of tiny energy shifts in the electrons’ behavior. By measuring these shifts, the team was able to infer information about the internal structure and magnetization distribution of the nucleus.
This kind of measurement is typically only possible in large-scale facilities like CERN, where high-energy particle accelerators smash electrons into nuclei. But this new molecule-based approach is a tabletop experiment, done in a small laboratory setup. That’s a big deal because it dramatically reduces the complexity, cost, and scale of nuclear probing experiments.
Step-by-Step: What the Researchers Did
Here’s how the team pulled it off:
- Creating the molecules: Researchers first produced tiny amounts of radium monofluoride. This is no small feat — radium is radioactive and has a short lifetime, so making stable molecules from it is extremely challenging.
- Trapping and cooling: Once formed, the RaF molecules were trapped in a series of vacuum chambers and cooled down to reduce random motion.
- Laser spectroscopy: They then used carefully tuned lasers to excite the molecules. As the lasers interacted with the electrons orbiting the radium atom, the researchers could measure their energy levels with extreme precision.
- Detecting the energy shift: When the results came in, the team noticed that the electron energies didn’t match what existing theory predicted. The only explanation was that the electrons were briefly entering the nucleus and interacting with its internal magnetic structure.
The observed energy shift was incredibly small — just one-millionth of the energy of the laser photon used to excite the molecule. But even such a minuscule change was enough to confirm that the electrons had sampled the inside of the nucleus.
This is the first clear proof that it’s possible to measure what’s going on inside an atomic nucleus using molecular spectroscopy.
The Role of Magnetization and Symmetry
Inside every nucleus, protons and neutrons each act like tiny magnets due to their intrinsic spin. How these “nuclear magnets” are distributed and aligned tells us about the magnetic structure of the nucleus.
In most atoms, this distribution is symmetrical — their nuclei are roughly spherical. But the radium nucleus is special. It’s pear-shaped, meaning it’s slightly asymmetric in charge and mass. This asymmetry makes it an ideal candidate for studying violations of fundamental symmetries, such as those involving time reversal or parity.
Physicists are deeply interested in these symmetry violations because they might explain one of the greatest mysteries in cosmology: why the universe contains much more matter than antimatter.
According to the Standard Model of particle physics, matter and antimatter should have been produced in nearly equal amounts during the Big Bang. But the universe we observe today is overwhelmingly made of matter. Something — some yet-undiscovered physical mechanism — must have tipped the balance.
By studying radium’s asymmetric nucleus, scientists hope to uncover new symmetry-violating effects that could help explain this imbalance.
Why Radium Is So Important
Most atomic nuclei are perfectly round or slightly deformed, but radium’s pear-shaped nucleus makes it uniquely valuable for these kinds of studies. Its unusual asymmetry acts as an amplifier for tiny symmetry-breaking effects that might otherwise be impossible to detect.
This means radium-based molecules could be among the most sensitive systems in the world for detecting new physics beyond the Standard Model.
The team’s method of using molecules like RaF offers a new, elegant way to access the nuclear interior without smashing it apart. Instead of destructive collisions, this approach relies on subtle energy measurements that reflect the internal arrangement of particles.
What the Results Mean
The MIT-led team’s measurements provide direct evidence that electrons can carry information from inside the nucleus to the outside world. That’s like having a messenger go into a locked vault, grab a piece of data, and bring it back out safely.
By analyzing these energy shifts, the researchers could reconstruct details about how the nuclear magnetization — the internal alignment of the protons’ and neutrons’ magnetic moments — is distributed within the radium nucleus.
These measurements pave the way for a completely new category of experiments. In the future, scientists could:
- Map the distribution of forces and magnetization inside other heavy nuclei.
- Study potential violations of fundamental symmetries directly within nuclei.
- Refine models of atomic structure that link nuclear physics and quantum chemistry.
This experiment essentially bridges two fields — nuclear physics and molecular spectroscopy — that usually operate in very different regimes.
Challenges and Future Plans
There are still major challenges ahead. Producing radium monofluoride molecules is difficult and can only be done in tiny quantities. Radium’s radioactivity and short lifetime make every experiment delicate and time-limited.
Moreover, the researchers currently work with molecules that have random orientations of their nuclei. To fully map the internal nuclear structure, they’ll need to cool the molecules further and control the orientations of those pear-shaped nuclei. That would let them probe specific nuclear directions and produce detailed, high-resolution maps of internal magnetization.
The team also hopes to apply this method to other exotic atoms and isotopes that could reveal even more about the subatomic world.
How This Compares to Traditional Nuclear Experiments
Traditional nuclear physics experiments usually involve massive particle accelerators that stretch for kilometers, such as those at CERN. These machines accelerate electrons or protons to near-light speeds and smash them into atomic nuclei to study the resulting debris.
While such experiments have been invaluable, they’re also complex, expensive, and limited to large-scale facilities. The molecule-based method developed by the MIT team offers a striking contrast:
- It can be done on a tabletop setup using controlled molecules.
- It’s non-destructive, since the nucleus remains intact.
- It opens the door to studying short-lived, radioactive isotopes that would otherwise be inaccessible.
This makes the technique especially attractive for studying exotic nuclei that exist only fleetingly in laboratory conditions.
The Bigger Picture: Why Understanding the Nucleus Matters
The atomic nucleus is at the core of everything — literally. It holds almost all the mass of ordinary matter and dictates the properties of elements, from hydrogen to uranium. Yet, despite decades of research, we still don’t fully understand how protons and neutrons behave inside it.
Unraveling the structure of the nucleus has implications that go far beyond basic science. It could lead to better nuclear models, improved atomic clocks, and new ways to test the limits of quantum theory.
And at the deepest level, it could help physicists answer that haunting cosmic question: Why does our universe exist as it does — filled with matter and not annihilated by antimatter?
By peering into the pear-shaped nucleus of radium, we may finally start to see the faint fingerprints of the physics that shaped our entire universe.
A Brief Background on Radium and Molecules Like RaF
Radium is a radioactive element discovered by Marie and Pierre Curie in 1898. It’s best known for its historical use in luminous paints and early cancer treatments, though its intense radioactivity later made those applications dangerous.
Radium sits in the same chemical family as barium and calcium but is far heavier. Because of its large nucleus and strong internal electric fields, it’s particularly sensitive to subtle nuclear effects that lighter atoms can’t display.
When bonded with fluorine to form radium monofluoride, the resulting molecule amplifies these effects even further. This makes RaF a perfect candidate for probing deep nuclear properties — essentially acting as a natural microscope for the atomic nucleus.
What Happens Next
The MIT group, led by Ronald Fernando Garcia Ruiz, plans to continue refining the technique. They want to:
- Cool the molecules to ultra-low temperatures to stabilize them.
- Align the pear-shaped nuclei to better control how electrons interact with them.
- Explore other heavy molecules that might show even stronger internal effects.
Their ultimate goal is to use this approach to search for symmetry violations that could reveal new physics beyond what the Standard Model predicts.
If successful, this could change how scientists study atomic nuclei and open a whole new path toward understanding the forces that built our universe.
Research Reference:
Wilkins, S. G., et al. (2025). Observation of the distribution of nuclear magnetization in a molecule. Science. DOI: 10.1126/science.adm7717