Scientists Develop a Superconducting Semiconductor Using Gallium-Doped Germanium
For decades, scientists have tried to make semiconductors that can also act as superconductors—materials that conduct electricity with zero resistance. This dream has now taken a big step forward. A team of researchers from New York University, the University of Queensland, and international collaborators has created a new form of germanium that becomes superconducting when heavily infused with gallium. Their work, recently published in Nature Nanotechnology (October 2025), could reshape the future of quantum technology, low-power electronics, and superconducting circuits.
The Breakthrough in Simple Terms
Germanium (Ge) is already one of the most important semiconductors used in computer chips, solar cells, and fiber optics. It belongs to Group IV on the periodic table, alongside silicon—the foundation of most modern electronics. But germanium, like silicon, doesn’t naturally become superconducting under normal conditions. That’s why this discovery is such a big deal.
The researchers succeeded in creating a superconducting form of germanium that can conduct electricity without energy loss. This happens at an extremely low temperature of 3.5 Kelvin, which is around -453 degrees Fahrenheit. While that’s far from room temperature, it’s still an exciting result because it shows that superconductivity can emerge in a material already central to semiconductor manufacturing.
How They Did It
To make germanium superconducting, the scientists used a process known as hyperdoping—injecting very high concentrations of another element into a crystal lattice to change its properties. In this case, they infused the germanium with large amounts of gallium (Ga), a soft metal commonly used in LEDs, sensors, and transistors.
Typically, pushing so much dopant into a semiconductor breaks the crystal structure, making the material unstable and useless. However, the researchers developed a new growth method that maintained crystal integrity even at extremely high doping levels. Instead of using ion implantation—a method that often damages the lattice—they relied on molecular beam epitaxy (MBE). This precise technique allows atoms to settle gently onto the surface, layer by layer, forming ultra-pure and uniform films.
With this method, the scientists achieved up to 17.9 percent gallium substitution within the germanium lattice—an incredibly high number that far exceeds the normal solubility limit (about 1.8 percent). The resulting material was not only stable but also showed superconductivity at low temperatures.
What Makes It Superconducting
The phenomenon of superconductivity arises when electrons in a material form pairs, known as Cooper pairs, that move together without resistance. In most metals, this requires cooling to very low temperatures so that lattice vibrations (phonons) can help bind the electrons into pairs.
Germanium doesn’t naturally provide the right conditions for this pairing. But by substituting enough gallium atoms into the germanium lattice, the researchers altered its electronic band structure. Essentially, they created a dense sea of mobile charge carriers (holes, in this case) that interact with the crystal in such a way that pairing becomes possible.
The team used advanced X-ray scattering and absorption techniques to confirm that the gallium atoms replaced germanium atoms at well-defined lattice sites instead of clumping together as metallic droplets. This substitutional arrangement, combined with slight tetragonal distortions in the crystal structure, created the perfect environment for superconductivity to emerge.
The Role of Molecular Beam Epitaxy
The use of molecular beam epitaxy is a central reason this experiment succeeded. This method allows scientists to grow epitaxial thin films—single-crystal layers that match the atomic structure of the underlying substrate. The precise control over temperature, deposition rate, and atomic flux ensures that the gallium atoms end up in the right positions within the lattice.
In the past, other researchers tried to achieve superconductivity in semiconductors using techniques like ion implantation or flash annealing, but those methods usually resulted in polycrystalline or highly disordered films. The new epitaxial approach avoids those problems, producing an ordered, stable film where superconductivity can truly develop.
Why This Matters
The implications of this breakthrough go beyond pure physics. Because germanium is already used in semiconductor foundries, this discovery could lead to scalable, foundry-ready superconducting devices. In other words, the same infrastructure that fabricates today’s chips might one day produce quantum circuits and cryogenic electronics using this new material.
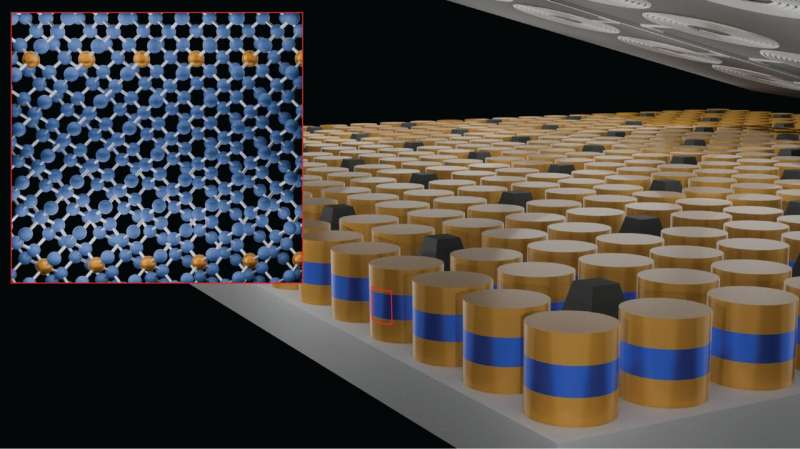
The research team demonstrated that millions of Josephson junctions—tiny quantum devices made of two superconductors separated by a thin non-superconducting barrier—can be created on a wafer-scale germanium substrate. Each junction can act as a pixel or a component in quantum computers, sensors, or superconducting detectors.
If this can be refined and commercialized, it might enable a new class of hybrid devices that merge semiconductor versatility with the efficiency of superconductors.
The Science Behind Group IV Superconductivity
To appreciate how remarkable this is, it helps to understand the family germanium belongs to. Group IV elements like carbon, silicon, and germanium have diamond-like crystal structures. They typically behave as semiconductors, meaning they can either conduct or insulate depending on temperature, impurities, and structure.
By contrast, superconductors—like niobium or lead—are usually metallic and have free electrons. Achieving superconductivity in nonmetallic, covalently bonded elements like germanium has long been thought nearly impossible without causing severe disorder. The ability to induce superconductivity in a crystalline, ordered semiconductor challenges that assumption and bridges two worlds that rarely overlap.
Interestingly, there have been other Group IV superconductors. Boron-doped diamond and silicon doped with boron or aluminum have shown weak superconductivity before, but their transition temperatures were much lower—often below 1 Kelvin. The new gallium-doped germanium surpasses those results both in critical temperature and material quality.
Limitations and Next Steps
Of course, the new material isn’t without challenges. Operating at 3.5 Kelvin means it requires liquid helium or closed-cycle cryocoolers—not something you can put in a laptop anytime soon. Still, for quantum computing, deep-space electronics, and research applications, those conditions are already standard.
The next step will be testing the durability, reproducibility, and device performance of this superconducting germanium under practical conditions. Scientists need to see how it behaves under magnetic fields, high current densities, and when patterned into real circuits.
Another big question is the mechanism itself. Is the superconductivity entirely phonon-mediated (like conventional superconductors), or do the gallium-induced distortions introduce new electronic effects? Answering that could lead to further tuning of the material’s properties—or even higher transition temperatures.
Potential Applications
The possibilities are wide-ranging.
- Quantum computing: Superconducting semiconductors could lead to more compact and stable qubits, reducing noise and improving coherence.
- Cryogenic electronics: Circuits that operate at near-zero resistance can drastically cut power consumption in low-temperature systems.
- Integrated sensors: Combining superconductivity and semiconductor behavior could yield ultra-sensitive magnetic or photonic sensors.
- Wafer-scale Josephson arrays: The ability to fabricate millions of junctions could be valuable for imaging systems, quantum simulators, and superconducting logic devices.
Putting It All Together
This discovery shows that superconductivity and semiconductor technology are not as separate as we once thought. By mastering atomic-scale control and pushing doping to extreme levels, scientists have made germanium—a familiar, workhorse material—do something extraordinary.
Even if we’re still decades away from superconducting smartphones, this achievement lays the groundwork for quantum-ready, energy-efficient electronics built from materials the tech industry already understands. It’s a reminder that innovation doesn’t always come from brand-new elements—it can come from reinventing what we already have.
Research Paper: Superconductivity in substitutional Ga-hyperdoped Ge epitaxial thin films – Nature Nanotechnology (2025)