Self-Organizing Plasma Patterns Show New Promise for Destroying PFAS in Water
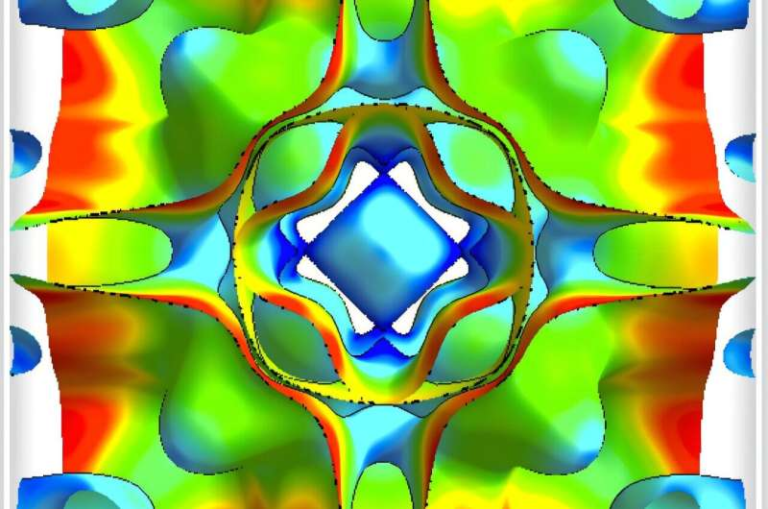
Researchers at the University of Michigan have uncovered fascinating new behavior in cold plasma that could make it far more effective at destroying PFAS, the notoriously stubborn “forever chemicals” contaminating water worldwide. Their findings reveal that when plasma interacts with water under specific conditions, it forms self-organized patterns—complex shapes like stars, wagon wheels, and gears—that expand the plasma’s contact area with the water. This larger interface could be exactly what’s needed to scale up plasma-based water treatment in an efficient, practical way.
The work appears in the journal Plasma Sources Science and Technology and offers some of the clearest real-time observations to date of how plasma physically shapes the water beneath it. The team even managed to capture these interactions at microsecond timescales, giving researchers rare insights into a plasma-water interface that transforms too quickly for the naked eye.
Below is a clear breakdown of the study, the physics behind it, why it matters for PFAS destruction, and additional context about plasma technologies and PFAS contamination itself.
What the Researchers Discovered
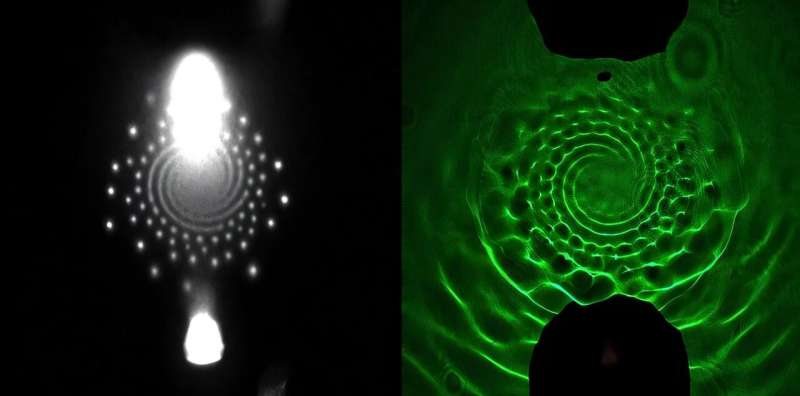
The team placed a cold plasma jet just a few millimeters above a water surface and observed something unusual. Instead of behaving like a diffuse glow, the plasma spontaneously arranged itself into intricate patterns—highly structured shapes that grew more complex as they spread outward.
Unlike ripples on water that naturally dissipate, these plasma patterns actually increase in complexity over time, behaving in what the researchers describe as “entropy-reversing” ways. More structure means more surface area, and more surface area means more opportunity for the plasma to react with contaminants in the water.
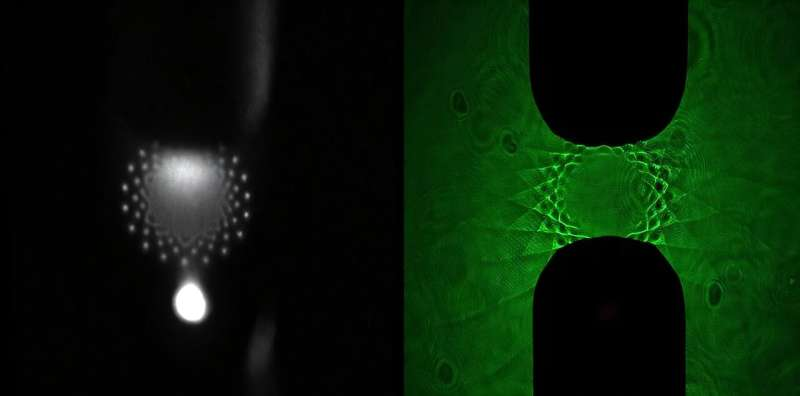
To better understand what was happening, the researchers captured the first ever images of the water’s surface directly beneath these patterns. They found that the plasma exerts an electrical force that pushes down on the water, mirroring the pattern’s shape on the water itself. These indentations also produce surface waves, which evolve depending on:
- the heating rate of the plasma jet
- the gas flow feeding the plasma
- the electrical properties of the water
By tuning these parameters, the contact area between plasma and water can potentially be expanded on demand, offering a scalable route for industrial water treatment.
Why PFAS Are So Hard to Destroy
PFAS (per- and polyfluoroalkyl substances) are synthetic chemicals used in products ranging from firefighting foam to non-stick cookware and stain-repellent coatings. They are engineered to resist heat and chemical breakdown, which also means they do not naturally degrade in the environment. Their defining feature—the exceptionally strong carbon-fluorine bond—gives them persistence but also makes them notoriously difficult to treat once they enter:
- groundwater
- rivers and lakes
- soil
- the food chain
Over time, PFAS accumulate in human and animal tissues and have been linked to cancers, immune system suppression, and endocrine disruption.
Traditional water treatment systems can filter or adsorb PFAS, but they can’t destroy them. This often means handling toxic waste afterward, rather than eliminating the pollutant.
How Cold Plasma Breaks PFAS Apart
Cold plasma isn’t hot like a flame—it’s created using high-voltage pulses that energize electrons without heating the surrounding gas or water. That’s why the water doesn’t boil or evaporate despite intense activity at the molecular level.
When the plasma touches water, it generates a cocktail of energetic species, including:
- ions
- solvated electrons
- excited molecules
- UV light
- ultrasound waves
- shockwaves
These energetic components can break the powerful carbon-fluorine bond in PFAS, dismantling the molecular structure. The process can fully mineralize PFAS into harmless substances such as fluoride ions and small carbon fragments.
Laboratory tests have shown that cold plasma can eliminate contaminants in water almost completely, but the challenge has always been scaling up the process without wasting energy.
This new discovery of controllable self-organized patterns could solve that.
Scaling Up the Technology
The key limitation of plasma-based PFAS treatment is energy consumption. Plasma requires significant power, and it typically affects only a small region directly below the jet. Expanding the contact area naturally—without proportionally increasing energy use—could make this technology viable at municipal or industrial scale.
The U-M findings suggest that by fine-tuning:
- gas flow
- electrical characteristics of the water
- heating rates of the plasma jet
researchers may guide the formation of larger and more efficient plasma patterns, boosting treatment speed and reducing cost.
If this can be controlled reliably, future systems could integrate plasma reactors directly into large-scale water treatment plants.
How the Team Captured Microsecond Interactions
The plasma-water interface evolves in about 10 microseconds, far too fast for conventional imaging. So the team built a specialized setup:
- a plasma jet positioned millimeters above water
- a green speckled laser aimed down at the water from an angle
- a high-speed camera synchronized with the plasma pulses
By capturing images at precisely timed intervals, they verified that the plasma patterns cause the water deformations, not the other way around.
Different experimental runs showed different wave shapes depending on how the plasma and water were tuned. These waves expand outward from the pattern boundary and could help distribute reactive species more widely within the water.
Additional Context: What Self-Organizing Patterns Mean in Physics
Self-organization occurs when a system naturally forms ordered structures without external shaping forces. It’s seen in phenomena like:
- sand dunes
- chemical reaction fronts
- honeycomb convection cells
- snowflakes
In this case, the plasma-water system behaves like an open thermodynamic system. Reactive species deposited into the water don’t accumulate to equilibrium levels, making it possible for order to emerge spontaneously. The plasma patterns become larger, more defined, and more structured as they spread—exactly the opposite of most natural dissipative patterns.
Harnessing this behavior for engineering applications is still new territory, and this research opens an interesting frontier for environmental cleanup.
A Quick Look at Plasma Medicine
One detail worth noting: cold plasma is already gentle enough to be used on biological tissue. The field of plasma medicine uses similar kinds of plasma jets for wound healing, sterilization, and other biomedical applications. This helps illustrate how plasma can remain “cold” while producing highly energetic electrons capable of powerful chemical reactions.
The same properties that make cold plasma effective in sterilization are what allow it to break some of the strongest chemical bonds known in organic chemistry.
A Wider Perspective on PFAS Cleanup Technologies
Many technologies are being explored for PFAS removal, each with strengths and weaknesses:
- Activated carbon filtration – widely used, but does not destroy PFAS
- Ion-exchange resins – effective but expensive and still produce toxic waste
- Advanced oxidation – limited success on PFAS
- Electrochemical oxidation – promising but energy-intensive
- Supercritical water oxidation – highly effective but requires extreme temperatures/pressures
- Plasma treatment – capable of destroying PFAS at atmospheric pressure without extreme heat
The main hurdle for plasma has been limited contact area and energy cost. This new research directly targets both of those issues, making it particularly exciting.
Why This Study Matters
The implications of this research reach beyond PFAS:
- It provides a deeper understanding of plasma-liquid interactions, one of the most complex areas in plasma physics.
- It reveals ways to naturally increase the reactive interface between plasma and water.
- It suggests future plasma reactors could treat larger water volumes more efficiently.
- It demonstrates a method for capturing microsecond-scale surface interactions.
Most importantly, it offers a path toward a practical, scalable solution for one of the world’s toughest water contaminants.
Research Paper
Surface deformation coupled with self-organized pattern on a liquid anode of 1 atm DC glow discharge
https://doi.org/10.1088/1361-6595/ae0764