A Tiny 3D-Printed Device That’s Changing the Future of Tissue Engineering
Imagine holding a piece of technology no bigger than your fingertip, yet powerful enough to redefine how scientists build human tissues in the lab. That’s exactly what researchers at the University of Washington and UW Medicine have developed: the STOMP device—short for Suspended Tissue Open Microfluidic Patterning.
This little 3D-printed tool is being celebrated as a big leap forward in tissue engineering, giving scientists the ability to arrange living cells with unmatched precision. For anyone fascinated by medical innovation, this breakthrough is a reminder of how far we’ve come in blending biology with cutting-edge engineering.
Why Tissue Models Matter
The ultimate goal of tissue engineering is to recreate environments where cells behave as they naturally would inside the body. These models help researchers study diseases, test treatments, and better understand how our tissues function.
Traditionally, one popular approach has been to suspend cells in a gel placed between two posts. This method has successfully grown tissues like skin, heart, lung, and muscle. While effective, it has limitations—especially when it comes to studying interactions between different tissue types. For example, modeling complex conditions like neuromuscular disorders requires much finer control than current systems allow.
That’s where STOMP shines.
How STOMP Works
Think of making Jell-O with fruit inside. Normally, you pour the mix into a mold and the fruit pieces float around, not always ending up where you want them. STOMP takes that idea and supercharges it with science.
Instead of pouring, researchers use capillary action—the same phenomenon that makes water climb up a straw. This allows them to guide different types of cells into very specific positions inside a gel. It’s like arranging fruit in Jell-O with pinpoint accuracy.
This simple yet powerful approach makes it possible to create distinct regions of tissue—say, healthy heart cells right next to fibrotic (diseased) ones—without adding complicated tools or steps.
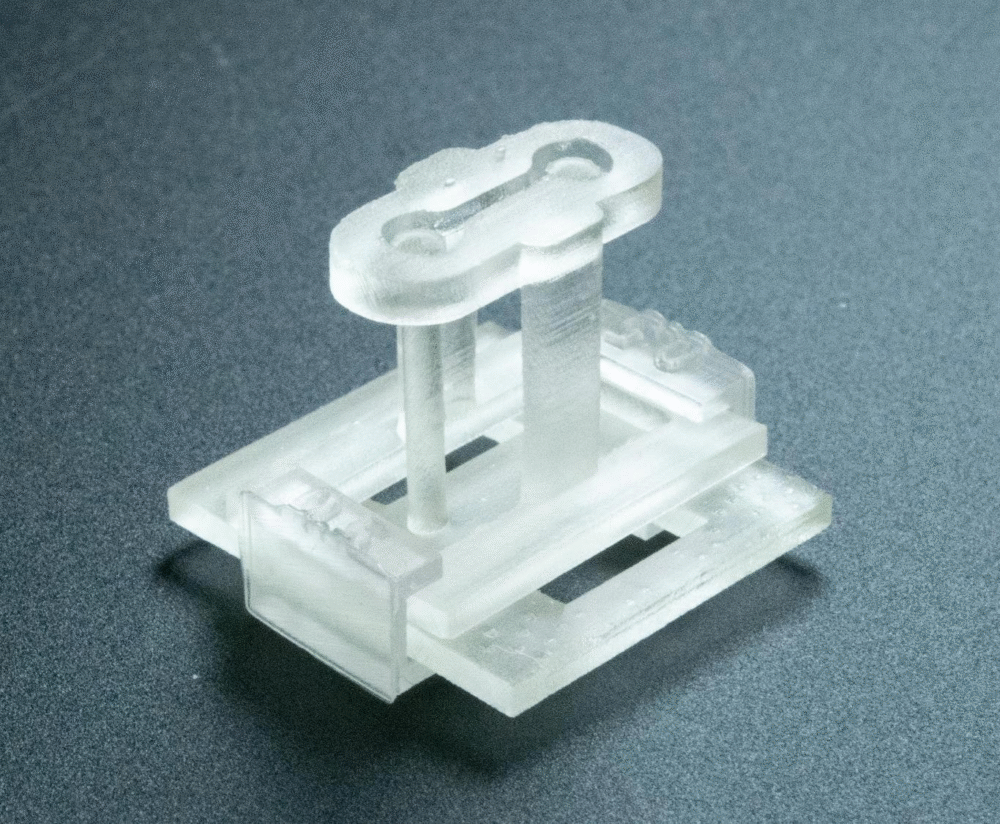
Small Device, Big Impact
The STOMP device itself is tiny, fitting neatly on a fingertip. It attaches to an existing two-post platform designed to measure how heart cells contract. Within this small setup lies an open microfluidic channel, carefully designed with geometric features that determine where cells go and how they’re spaced.
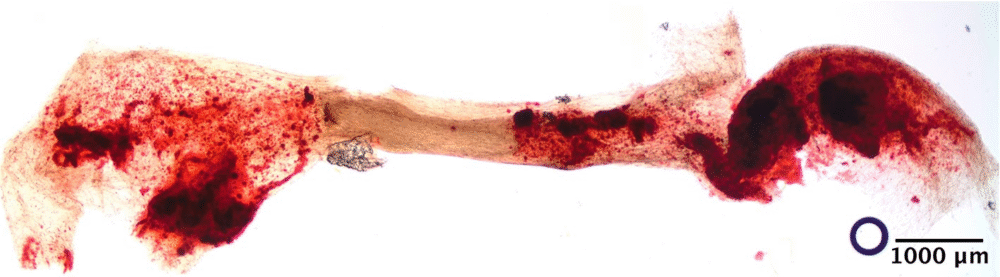
Researchers put it to the test in two experiments:
- Studying the contraction of healthy and diseased heart tissue side by side.
- Recreating the ligament that secures a tooth inside its socket.
In both cases, STOMP delivered results that showed just how powerful precise tissue modeling can be.
Added Versatility
Another clever feature comes from the use of degradable hydrogel walls. These walls can be broken down once the tissue is formed, leaving behind a freestanding tissue structure. That flexibility means scientists can work with weaker cell types or more delicate biomaterials that wouldn’t hold up in traditional molds.
This “nonstick” quality adds a whole new level of versatility to tissue engineering—making it possible to model even more scenarios than before.
The Team Behind the Innovation
The project was led by UW professors Ashleigh Theberge (chemistry) and Nate Sniadecki (mechanical engineering), along with contributions from students and faculty across chemistry, bioengineering, dentistry, and medicine. Their collaboration highlights how solving tough scientific challenges often requires expertise from many different fields.
Funding came from a mix of the National Institutes of Health (NIH), UW, and several foundations focused on muscular dystrophy and other diseases.
Looking Ahead
The researchers believe that STOMP could open doors to studying cell signaling, disease progression, and tissue repair in ways that were previously out of reach. It’s not just about building better tissue models—it’s about giving scientists the freedom to ask new questions and explore new frontiers in medicine.
In other words, this fingertip-sized device might just change how we understand and treat complex diseases in the future.





