Artificial Cartilage That Delivers Drugs on Demand Could Transform Arthritis Treatment
Researchers at the University of Cambridge have created a new type of cartilage-like material that doesn’t just mimic the cushioning function of natural cartilage but also acts as a smart drug delivery system. This breakthrough could completely change how arthritis—and possibly other inflammatory diseases—are treated in the future.
The material is designed to sense tiny chemical changes in the body, especially the rise in acidity that comes with inflammation, and respond by releasing medication exactly where and when it’s needed.
How the Smart Material Works
Inflamed joints, like those seen during an arthritis flare-up, often become slightly more acidic compared to healthy tissue. The Cambridge team developed a gel-like hydrogel that can detect these subtle pH shifts. When the environment around the material becomes more acidic, the gel changes its texture, becoming softer and more flexible. This change triggers the release of drugs stored inside the material.
The science behind this process is called kinetic locking. The material is built from a polymer network cross-linked by special chemical structures known as host–guest complexes. At a normal, healthy pH, these complexes allow the components to move relatively freely. However, when acidity increases, the chemical interactions change: the negative charge from carboxylic acid groups causes the guest molecules to become locked inside their host structures. This “locking” alters the mechanics of the material, softening it and allowing the controlled release of drugs.
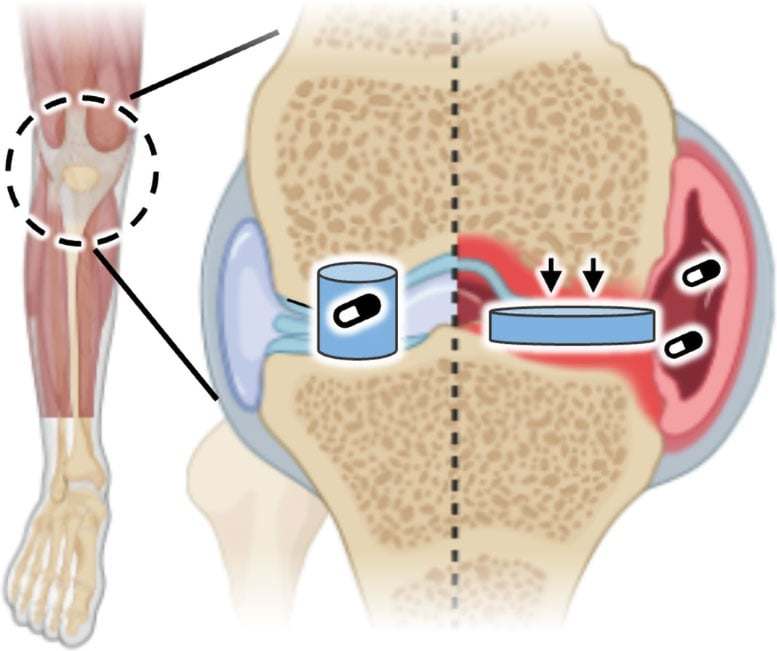
This property makes the material behave like artificial cartilage—able to cushion joints while also serving as a smart, responsive treatment tool. Unlike other drug delivery methods that require external triggers such as light, heat, or magnets, this material relies entirely on the body’s own chemistry.
Why This Matters for Arthritis
Arthritis is a condition that affects more than 10 million people in the UK and an estimated 600 million people worldwide. In the UK alone, the cost to the National Health Service (NHS) is believed to be around £10.2 billion annually. Standard treatment usually involves oral anti-inflammatory drugs, injections, or surgery in severe cases. However, these treatments often come with side effects, especially when medication circulates throughout the entire body.
This new material could allow for local, targeted, and on-demand drug release, drastically improving how arthritis flare-ups are managed. Instead of taking repeated doses of medication or undergoing frequent injections, patients could rely on a single implant that automatically dispenses medication only when inflammation occurs. That means fewer side effects, more consistent relief, and potentially improved quality of life.
Lab Testing and Results
To test the system, the researchers infused the material with a fluorescent dye, simulating how an actual drug might behave. When the material was placed in an acidic environment—similar to that found in inflamed arthritic joints—it released significantly more of the dye than when tested in normal pH conditions.
These results demonstrate that the material is highly sensitive to small pH changes and can reliably deliver medication when and where it is required. The tunable chemistry of the gel also means that researchers can adjust how sensitive it is and how fast it releases its cargo. In theory, the material could be designed to release drugs rapidly during sudden flare-ups, or slowly over weeks and months for longer-term therapy.
Who Developed It
The breakthrough came from the lab of Professor Oren Scherman, who leads the Melville Laboratory for Polymer Synthesis in Cambridge’s Yusuf Hamied Department of Chemistry. The study was carried out by his research group, with contributions from Dr. Stephen O’Neill, Dr. Jade McCune, and others.
Their results were published in the Journal of the American Chemical Society in September 2025, under the title “Kinetic Locking of pH-Sensitive Complexes for Mechanically Responsive Polymer Networks.” The research was supported by the European Research Council and the Engineering and Physical Sciences Research Council (EPSRC), which is part of UK Research and Innovation (UKRI).
What Comes Next
While the lab results are promising, this technology is still in its early stages. The next big step is testing the material in living systems to ensure that it is safe, effective, and durable in real physiological conditions. Researchers need to confirm:
- Whether the material maintains its properties inside the body over time
- How long it can deliver drugs without breaking down
- How the immune system reacts to it
- The best way to implant it into joints
- Whether it can be reloaded or needs replacement after use
If successful, this technology could pave the way for long-lasting, self-regulating arthritis treatments.
Potential Beyond Arthritis
Although the focus right now is arthritis, the same principle could be applied to other chronic diseases. Many conditions are associated with localized changes in pH and inflammation, including certain types of cancer. By tweaking the chemical sensitivity of the material, researchers believe they could target a wide range of illnesses where smart, localized treatment would be more effective than systemic medication.
The Broader Field of Smart Biomaterials
This discovery is part of a larger movement in science toward smart biomaterials—materials designed not only to function passively inside the body but also to sense, adapt, and respond to their environment.
Other examples include:
- pH-sensitive nanoparticles that release chemotherapy drugs directly at tumor sites.
- Temperature-sensitive hydrogels that release insulin in response to spikes in blood sugar.
- Magnetically responsive materials that can be controlled externally to release drugs when needed.
What sets the Cambridge material apart is that it doesn’t rely on external controls. Instead, it uses the body’s own chemical signals to decide when to act, making it a more natural and potentially longer-lasting solution.
Challenges Ahead
While the promise is enormous, there are still plenty of challenges before patients could benefit from this technology:
- Practical implantation: Would this material be injected as a gel, or surgically implanted as a patch? How easy would it be to perform the procedure in multiple joints?
- Longevity: Arthritis is a lifelong condition. Will the material last for months, years, or require regular replacement?
- Multiple affected joints: For people with rheumatoid arthritis, inflammation can affect many joints at once. Would multiple implants be required?
- Safety and immune response: Any foreign material introduced into the body risks triggering an immune reaction. Ensuring biocompatibility is essential.
- Scalability: Developing this material in a lab is one thing. Producing it at large scale for widespread medical use is another.
These questions will only be answered through further preclinical and clinical trials, which will take years. But the fact that the material already shows clear laboratory success is an encouraging sign.
Why Acidity Matters in Disease
A key detail in this study is the reliance on acidity (pH levels) as a trigger for drug release. Many diseases, not just arthritis, cause localized acidity:
- Cancer tumors often generate acidic microenvironments due to rapid cell growth and metabolism.
- Infections can alter pH in tissues.
- Inflammatory conditions generally lead to small but significant drops in pH.
This makes pH a highly reliable biomarker for disease activity, and using it as a trigger for drug release is a clever and powerful approach.
A Glimpse Into the Future
Imagine a future where instead of taking pills daily, patients with arthritis or similar chronic diseases could receive a single implant that quietly works in the background. It would detect when a joint is becoming inflamed and release just enough medicine to stop the flare-up before it worsens. No excess drug in the bloodstream, no long-term exposure to side effects, and no need for repeated hospital visits.
While that vision may still be years away, this study shows that the technology is possible. It’s a promising reminder of how advances in chemistry and materials science are steadily transforming medicine.
Final Thoughts
This new artificial cartilage material represents an exciting step toward the development of smart, self-regulating therapies. By combining the mechanical properties of cartilage with the ability to sense chemical changes and deliver drugs, researchers are opening the door to a new era of treatments for arthritis and beyond.
If ongoing research confirms safety and effectiveness in living systems, we may one day look back on this as the moment when chronic disease treatment became truly responsive and personalized.