Chemists Achieve Low-Temperature Nanodiamond Growth Using Electron Beams
Researchers at the University of Tokyo have reported a breakthrough in synthetic diamond research: the successful conversion of adamantane, a carbon cage molecule, into defect-free nanodiamonds using a controlled electron-beam irradiation method. This new approach is significant because it bypasses the extreme pressures and temperatures normally required for diamond formation, offering a low-pressure and relatively gentle pathway for creating diamond structures at the nanoscale.
This achievement, published in Science, marks the culmination of two decades of work by Professor Eiichi Nakamura and his team. The method also demonstrates how electron beams, traditionally thought to destroy organic molecules, can instead drive well-defined chemical transformations under carefully tuned conditions.
Why Adamantane Was the Chosen Starting Point
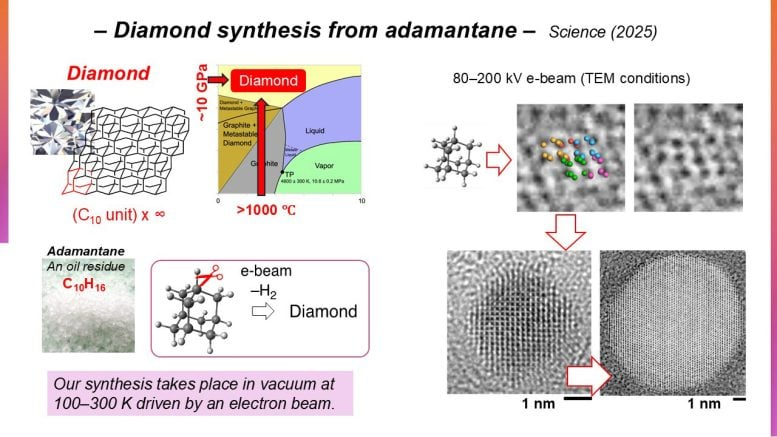
The starting material for this research was adamantane (C₁₀H₁₆), a highly symmetrical hydrocarbon. Adamantane is special because its molecular structure mirrors the arrangement of carbon atoms in a diamond lattice. Both share a tetrahedral framework with carbon atoms arranged in three-dimensional space in a nearly identical way.
This similarity makes adamantane a logical candidate for diamond synthesis. The challenge lies in selectively breaking its C–H bonds (carbon-hydrogen bonds) and replacing them with new C–C bonds (carbon-carbon bonds) so that the molecules link up into a true diamond lattice.
Previous research suggested that single-electron ionization could trigger such bond cleavage, but experimental verification was limited. Most methods destroyed organic materials rather than transforming them. This is where Nakamura’s group took a bold approach—using electron beams not just as an imaging tool but as a driver of controlled chemistry.
The Electron-Beam Irradiation Process
The team prepared tiny sub-microcrystals of adamantane and placed them under a transmission electron microscope (TEM) setup. They then irradiated the samples using electron beams at 80–200 kiloelectron volts (keV).
Key experimental conditions included:
- Temperature: 100 K to 296 K (from cryogenic conditions to room temperature).
- Vacuum environment: Ensuring controlled irradiation without contamination.
- Irradiation time: Tens of seconds to minutes, depending on the observation stage.
During irradiation, the adamantane molecules gradually lost hydrogen atoms. This C–H cleavage released hydrogen gas and allowed new carbon bonds to form. Over time, these rearrangements produced spherical nanodiamonds with a cubic crystal structure.
One of the most striking results was that the process produced defect-free nanodiamonds, meaning the crystal lattice was intact and free of structural irregularities. This is rare, especially under conditions where materials are expected to decompose.
Observing the Process in Real Time
What makes this work particularly exciting is that the team could actually watch the transformation happen using advanced TEM techniques. They employed time-resolved, atomic-resolution imaging to follow how adamantane molecules evolved.
The stages they observed were:
- Formation of adamantane oligomers – short molecular clusters emerging from partially linked adamantane units.
- Reorganization into nanodiamond seeds – the oligomers began adopting three-dimensional diamond-like ordering.
- Growth of spherical nanodiamonds – the seeds developed into nanodiamonds up to 10 nanometers in diameter under prolonged exposure.
These observations confirmed theoretical predictions and provided direct visual evidence of diamond lattice assembly at the nanoscale.
The Role of C–H Bond Cleavage
The researchers also conducted isotope experiments using deuterated adamantane (where hydrogen atoms were replaced with deuterium). They found that the rate of nanodiamond formation slowed significantly. This confirmed that C–H bond cleavage is the rate-determining step in the transformation.
Other hydrocarbons tested under the same conditions failed to produce nanodiamonds. This result highlights that adamantane’s unique geometry and symmetry are critical to the method’s success.
Nanodiamond Properties and Sizes
The resulting nanodiamonds were initially in the 2–4 nm range. With extended irradiation, larger structures formed, reaching 8–20 nm in size. Some nanodiamonds fused together to form twin crystals.
Importantly, the nanodiamonds were observed to be:
- Cubic in structure (the most stable diamond form).
- Highly spherical with good circularity.
- Free from defects within the lattice.
Such structural quality makes them highly attractive for scientific and technological applications.
Broader Implications of the Discovery
1. Moving Beyond Extreme Conditions
Traditional diamond synthesis methods include:
- High-pressure, high-temperature (HPHT) synthesis, which replicates Earth’s mantle conditions.
- Chemical vapor deposition (CVD), which deposits diamond layers from gas-phase carbon.
Both methods involve extreme environments and complex setups. By contrast, this electron-beam method works at low pressure and relatively low temperatures, making it conceptually much simpler.
2. Potential Applications
The findings open up possibilities in several areas:
- Quantum technology: Nanodiamonds can host color centers that serve as stable quantum bits (qubits) or nanoscale sensors.
- Advanced imaging: Defect-free nanodiamonds can be tagged for biological imaging without degrading under electron beams.
- Materials science: The method expands our understanding of how to use electron beams as a chemical tool, not just as a destructive probe.
- Geology and planetary science: The work supports theories that natural nanodiamonds in meteorites or uranium-rich rocks may form under particle irradiation in space.
3. A Paradigm Shift in Electron-Beam Use
Electron beams are widely used in microscopy and lithography. Until now, their reputation was primarily destructive toward organic materials. This study overturns that assumption, showing that electron irradiation can enable precise, constructive chemistry when starting with carefully designed molecules.
Challenges and Limitations
Despite its promise, the method comes with challenges:
- Scalability: The current process only makes nanodiamonds a few nanometers wide. Scaling this to produce bulk or gem-quality diamonds is far off.
- Precursor limitation: Other hydrocarbons failed, meaning this method may only work with adamantane or related cage molecules.
- Control of growth: Preventing nanodiamonds from fusing into irregular structures will require further research.
- Beam damage risk: While successful here, electron beams remain destructive under many conditions, limiting broader applicability.
The Long Road to Success
This breakthrough represents the culmination of 20 years of effort. Professor Nakamura had long suspected that electron beams could be harnessed for constructive chemistry rather than destruction. Since 2004, his team pursued this vision despite skepticism from the microscopy community.
By combining expertise in synthetic chemistry, computational modeling, and electron microscopy, the team achieved direct visual confirmation of diamond formation at the atomic scale.
Funding and Research Support
The research was supported by:
- Japan Society for the Promotion of Science (JSPS KAKENHI)
- JST PRESTO program
A patent application for the method has also been filed, pointing to future development possibilities.
What Are Nanodiamonds?
To give more context, nanodiamonds are tiny diamond particles, typically ranging from 2 nm to a few hundred nanometers. They have unique properties such as:
- High hardness and thermal conductivity, like bulk diamonds.
- Surface functionalizability, allowing them to be modified for drug delivery or sensing.
- Quantum defects, such as nitrogen-vacancy (NV) centers, useful in quantum computing and high-precision magnetic field detection.
Because of their nanoscale size, nanodiamonds find applications in biomedicine, materials engineering, quantum technology, and lubrication. The ability to create defect-free nanodiamonds in a controlled, low-energy process could therefore be transformative.
Looking Ahead
This discovery may not immediately revolutionize diamond production for jewelry, but it sets the stage for new experimental methods in chemistry, physics, and materials science. By proving that electron beams can construct, not just destroy, researchers now have a tool to explore similar transformations in other molecular systems.
The real excitement lies in the scientific foundation this method provides—shaping how we understand molecular stability under high-energy beams and inspiring new applications in nanotechnology and quantum devices.
Research Reference: Rapid, low-temperature nanodiamond formation by electron-beam activation of adamantane C–H bonds – Science