DNA Nanostructures Get a Major Upgrade With Ionic Liquids That Boost Stability and Targeting
DNA nanostructures have been gaining serious attention in recent years, especially in biomedical research. Built by carefully folding strands of DNA into precise shapes, these tiny structures are highly programmable and can be engineered for tasks like disease detection, drug delivery, and therapeutic targeting. However, despite their enormous promise, one major challenge has consistently held them back: they tend to fall apart quickly inside real biological environments.
Now, researchers at the University of Illinois Grainger College of Engineering have found a way around this limitation. Their new study shows that assembling DNA nanostructures using ionic liquids instead of traditional metal ions dramatically improves stability, durability, and even targeting performance. The work was led by bioengineering professor Xing Wang and graduate student Dhanush Gandavadi, and it was published in 2025 in the Journal of the American Chemical Society.
Why DNA Nanostructures Matter in Medicine
DNA nanostructures are not ordinary genetic material. They are carefully designed nanoscale frameworks created by folding DNA strands into specific shapes, often using a technique known as DNA origami. These structures can be customized to recognize specific molecules, bind to cell receptors, or carry therapeutic payloads.
Wang’s lab has previously used DNA nanostructures for rapid virus detection, potent inhibition strategies, and targeted cancer drug delivery. Their appeal lies in their precision: DNA can be programmed with near-molecular accuracy, making it an ideal material for next-generation biomedical tools.
But there has always been a catch.
The Magnesium Ion Problem
Traditionally, DNA nanostructures are assembled using magnesium ions. These ions help neutralize the negative charges on DNA strands, allowing them to fold and hold their shape. While magnesium is essential during assembly, it becomes a problem once the structures are placed in biological environments such as blood or cellular fluids.
Inside the body, magnesium-based DNA nanostructures are highly unstable. They degrade quickly due to enzymes, competing ions, and complex biochemical conditions. This rapid breakdown severely limits their usefulness for therapeutic or diagnostic applications, where stability over time is critical.
This instability issue has been one of the biggest barriers preventing DNA nanostructures from moving beyond laboratory experiments into real-world medical use.
Turning to Ionic Liquids for a Solution
To solve this problem, Wang and Gandavadi explored an alternative approach: ionic liquids. Ionic liquids are salts that remain in liquid form at or near room temperature. They have long been used in industries like petrochemicals, electrochemistry, and battery technology, but their use in biomedical nanotechnology has been limited.
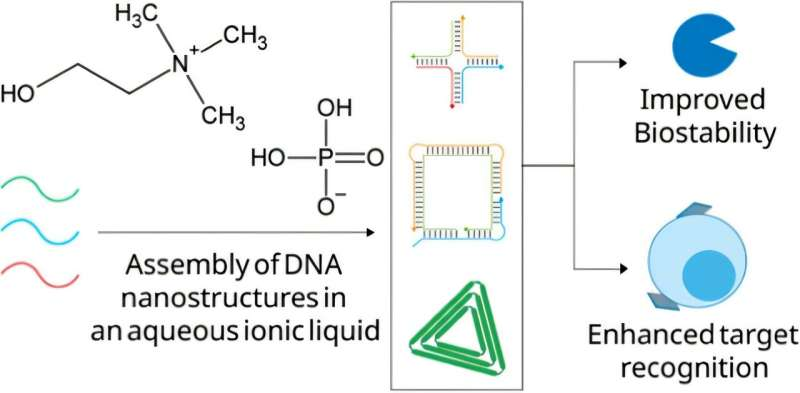
The team focused on an ionic liquid called choline dihydrogen phosphate (CDHP). Instead of using magnesium ions, they assembled DNA nanostructures directly in an aqueous solution of this ionic liquid.
This turned out to be a breakthrough.
The new method provided a simpler and faster assembly process while dramatically improving how DNA nanostructures behave in harsh biological conditions. The researchers described the approach as essentially giving DNA nanostructures a protective suit that allows them to survive and function inside real biological systems.
Improved Stability in Harsh Biological Environments
One of the most striking results of the study was how long the ionic liquid–assembled DNA nanostructures lasted. In simulated biological environments, the structures remained intact for up to 48 hours, a major improvement over magnesium-based systems, which typically degrade much sooner.
This enhanced stability comes from two key factors. First, assembling the nanostructures in CDHP changes how the DNA folds and interacts at the molecular level. Second, the free ionic liquid ions in solution provide ongoing protection against enzymatic degradation.
Together, these effects make the nanostructures far more resilient when exposed to conditions similar to those found inside the human body.
Better Target Binding and Cancer Cell Affinity
Stability was not the only improvement. The researchers also found that the ionic liquid–assembled DNA nanostructures showed stronger binding to protein biomarkers on the surfaces of cells.
This improved binding translates into more precise targeting, which is especially important for medical applications like cancer therapy. In fact, the study revealed that these nanostructures displayed a notable affinity for cancer cells, something that had been difficult to achieve consistently with magnesium-based assembly methods.
Enhanced targeting means that DNA nanostructures can more effectively recognize and attach to specific cells, increasing the chances of delivering drugs or diagnostic signals exactly where they are needed.
A Simpler and More Accessible Assembly Method
Another major advantage of the ionic liquid approach is its practicality. Many current DNA nanostructure assembly and stabilization strategies are complex, expensive, and multi-step. They often require additional coatings or chemical modifications that can reduce functionality under physiological conditions.
In contrast, the new method offers a one-step, cost-effective alternative. DNA nanostructures assembled in ionic liquids are both stable and functional without requiring extensive post-processing. This simplicity could make the technology far more accessible to labs around the world, not just highly specialized research groups.
Implications for Drug Delivery and Nanomedicine
Beyond DNA nanostructures themselves, the findings open the door to broader applications of ionic liquids in medicine. The study suggests that ionic liquids could act not only as protective environments but also as functional carriers for therapeutic nanomaterials.
This dual role—protecting the structure while enhancing biological interactions—is particularly exciting for drug delivery. Ionic liquid–based DNA nanostructures could potentially carry drugs more efficiently, remain stable in circulation longer, and bind more effectively to target cells.
Wang’s lab is already working on translating this platform toward cancer therapy, focusing on how these stabilized nanostructures perform when delivering payloads to cancer cells.
Collaboration and Broader Impact
The research also benefited from collaboration beyond Illinois. Professor Arun Richard at the University at Albany contributed by demonstrating that smaller, tile-based DNA motifs could also be assembled using ionic liquids. This suggests that the approach is not limited to one specific type of DNA nanostructure.
The broader implication is clear: ionic liquid–based assembly could become a general strategy across DNA nanotechnology, potentially reshaping how researchers design and deploy nanoscale biomedical tools.
Why This Matters for the Future of DNA Nanotechnology
DNA nanostructures sit at the cutting edge of biomedical innovation, but their real-world impact depends on overcoming practical limitations. By addressing stability, improving targeting, and simplifying assembly, this new ionic liquid approach removes several major roadblocks at once.
With greater durability, enhanced binding affinity, and lower barriers to adoption, DNA nanostructures may finally be ready to move from experimental systems into clinical and therapeutic applications.
The work from Wang’s team represents a meaningful step toward unlocking the full potential of DNA-based nanomedicine.
Research paper:
https://doi.org/10.1021/jacs.5c13969