Flatworm Stem Cells Rewrite the Rules of Regeneration
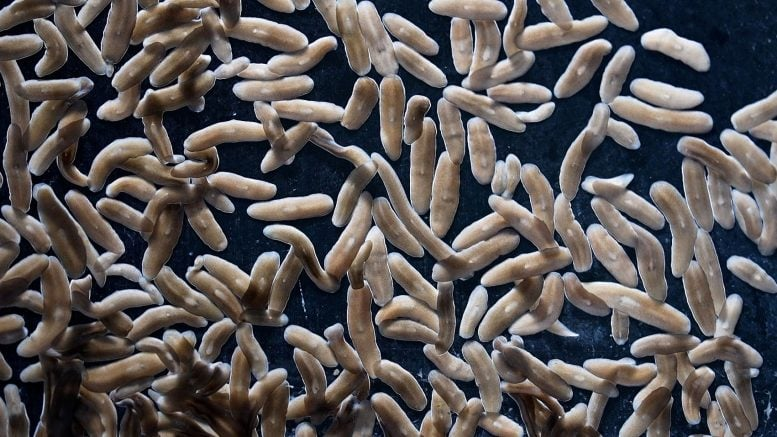
Scientists at the Stowers Institute for Medical Research have made a discovery that challenges one of the longest-standing ideas in stem cell biology. Their new study, published in Cell Reports on October 15, 2025, reveals that planarian flatworms—tiny, ribbon-like creatures famous for their astonishing ability to regrow lost body parts—use stem cells in a way that defies textbook biology.
Instead of relying on nearby “niche” cells for instructions, the flatworm’s stem cells appear to take regenerative cues from distant tissues, allowing them to rebuild entire organs or even an entire body from a tiny fragment. This discovery not only explains why these worms are almost immortal in their regenerative capacity but also provides new insight into how human stem cells might one day be controlled for tissue repair and regenerative medicine.
What Makes Planarians So Special
Before diving into the details of the study, it’s worth understanding why planarians are such extraordinary creatures. These flatworms, often found in freshwater environments, are biological marvels. If you slice one into several pieces, each fragment can regrow into a complete new worm—including its brain, eyes, digestive system, and all other organs.
This regenerative ability comes from a special type of stem cell called neoblasts. Neoblasts make up about 20% of the planarian’s body and have an almost limitless capacity to turn into any type of cell—neurons, muscle cells, intestinal cells, or reproductive cells. In humans, stem cells are usually more limited. For instance, our blood-forming stem cells can only make different kinds of blood cells. Planarians, on the other hand, seem to have found a biological loophole that keeps their regenerative powers wide open.
The Discovery That Changes the Textbooks
The new study, led by Dr. Frederick “Biff” Mann, a postdoctoral researcher at the Stowers Institute, and Dr. Alejandro Sánchez Alvarado, the institute’s president and chief scientific officer, has uncovered how planarian stem cells manage this remarkable feat.
For decades, scientists have believed that stem cells depend on their niche—a specialized environment where surrounding cells control how a stem cell divides, renews itself, or becomes a particular cell type. In humans, for example, blood stem cells live in the bone marrow, where they are tightly regulated by other nearby cells to prevent uncontrolled growth.
But the Stowers team found that in planarians, this idea simply doesn’t hold. Instead, the worm’s stem cells operate with a surprising level of independence, responding to cues that come from faraway parts of the body rather than from immediate neighbors. This finding suggests that the stem cells are not micromanaged by a fixed niche but can act freely, guided by a more flexible, body-wide communication system.
Peering Inside with Cutting-Edge Tools
To uncover these principles, the researchers used spatial transcriptomics, a technology that allows scientists to see which genes are turned on in specific cells while also mapping their positions in the body. This method gave the team a kind of “cellular map” of the flatworm—showing not only the stem cells but also all the other cell types that live around them.
By analyzing this map, the scientists discovered an unexpected neighbor to the stem cells: a large, complex cell type with many finger-like extensions, which they named hecatonoblasts. The name comes from Greek mythology, inspired by the hundred-armed giants called Hecatoncheires.
Because these hecatonoblasts were found so close to the stem cells, the team initially thought they must play a crucial role in controlling stem cell activity. But to their surprise, experiments showed that hecatonoblasts weren’t actually in charge. When their function was disrupted, the stem cells kept working normally, regenerating tissues just fine.
Distant Signals with Local Impact
If neighboring cells weren’t giving the orders, where were the instructions coming from? The researchers found their answer in an unexpected place: the intestine.
The intestinal cells, although located far from many of the stem cells, were found to send signals that direct stem cell activity during regeneration. This means that instead of a fixed local niche, planarian stem cells are tuned into long-distance communication networks within the body. These signals tell the stem cells where they are and what kind of tissue needs to be rebuilt.
This discovery paints a picture of a biological system that works more like a networked community than a hierarchy. The body’s cells seem to cooperate over long distances, coordinating regeneration at a systemic level rather than through local control alone.
Why This Matters for Humans
For humans and other animals, this finding could reshape how scientists think about stem cell control and tissue repair. Our own stem cells live in highly controlled environments to prevent them from “going rogue” and turning cancerous. But the flexibility of planarian stem cells shows that it’s possible to maintain high regenerative potential while avoiding uncontrolled growth.
If researchers can learn how planarian stem cells stay both powerful and disciplined, we might one day be able to reprogram human stem cells to regenerate organs or repair injuries more effectively. Understanding how long-range signals control regeneration could also help develop new treatments for diseases where tissues degenerate, such as heart failure or neurodegenerative disorders.
The Risk of Going Rogue
One important reason human stem cells are tightly regulated is to prevent cancer. Unchecked cell growth can quickly turn deadly, so our biology has evolved to keep stem cell activity under strict supervision. In planarians, however, even though their stem cells have virtually no limits, they rarely form tumors. This means that they’ve evolved a way to maintain regeneration without losing control.
Dr. Sánchez Alvarado and his colleagues believe that by studying how planarians manage this balance—staying both potent and disciplined—we might uncover basic biological “rules” that apply across species. Learning these rules could one day help scientists create safer, more effective regenerative therapies for humans.
The Dynamic World of Stem Cell Niches
Another major insight from this study is that the planarian stem cell environment is not static. It’s dynamic, changing as the worm grows, regenerates, or repairs itself.
In traditional models, a niche is thought of as a stable place—a fixed set of cells that continuously provide instructions. But in planarians, the niche seems to form and reform depending on what’s needed. The stem cells appear to make “friends” as they go, forming temporary partnerships with surrounding cells that guide their journey from a stem cell to a specialized cell type.
This flexible system might be one of the reasons why planarians can regenerate so effectively. Instead of being confined to a single niche, their stem cells can adapt to different environments, taking cues from a variety of sources as they rebuild tissues.
Understanding Spatial Transcriptomics
The study’s success relied heavily on spatial transcriptomics, a cutting-edge method that’s transforming biology. Traditional genetic analysis can tell scientists which genes are active, but it doesn’t show where they are active. Spatial transcriptomics combines gene expression data with precise spatial maps, showing how different cell types communicate in their native context.
Using this technology, the researchers could see not only which genes the planarian stem cells were using but also how those genes related to surrounding tissues. This approach made it possible to detect the surprising influence of distant intestinal cells and to identify the mysterious hecatonoblasts.
A Step Toward Regenerative Medicine
Although planarians and humans are worlds apart evolutionarily, studies like this provide valuable clues about what it might take to engineer regeneration in higher organisms. Understanding that stem cells can respond to distant cues suggests new ways to design bioengineered tissues or scaffolds that replicate these communication networks.
In the future, it might be possible to create regenerative systems where human stem cells receive global body signals—such as hormones, metabolic cues, or tissue damage alerts—similar to how planarian cells do. This could help repair large-scale injuries or degenerative damage more effectively than local therapies.
What’s Next
The Stowers Institute team plans to continue exploring how intestinal cells send long-distance signals and which molecules carry these messages. They also hope to study how the planarian’s body coordinates regeneration across different tissues simultaneously, ensuring that the new parts grow in perfect proportion.
As the researchers point out, the more we understand how stem cells interpret both local and distant signals, the closer we get to unlocking the regenerative potential hidden in all animals, including humans.
The Broader Lesson
The discovery doesn’t just expand our knowledge of flatworms—it forces biologists to rethink fundamental concepts in stem cell science. It suggests that the traditional idea of a “niche” might be too limited, at least for some organisms.
Instead of seeing the niche as a fixed location, it may be better to view it as a flexible, dynamic network—one that can adapt to the needs of the organism, allowing regeneration and repair on a much larger scale.
For the planarian flatworm, this flexibility has given it a near-immortal ability to regenerate. For humans, understanding it could one day mean the ability to grow back what was once lost.
Reference
Research Paper: Molecular and cellular characterization of planarian stem cell microenvironments – Cell Reports (October 15, 2025)