Four Distinct Antibody Response Paths After COVID Vaccination — And What It Means for You
Imagine two people getting their COVID-19 booster shots on the same day. Both mount a solid immune response at first. But six months later, one remains well-protected while the other ends up infected. A new Japanese study helps explain exactly how that can happen—by showing that how your immunity fades over time matters just as much as how strong it rises.
What This Study Did
Researchers at Nagoya University followed 2,526 participants in Fukushima, Japan over 18 months to observe their antibody responses from the initial vaccination through one booster.
They collected repeated blood samples over that period and used mathematical modeling + machine learning to classify different patterns of immune response. (Nagoya University)
Their goal: rather than just measuring antibodies at a single time point, track how those levels change over time, and see whether those trajectories predict who gets breakthrough infections. (Science)
They also looked at IgA (spike-specific IgA in blood, and in a subset nasal IgA), because IgA is a key antibody class protecting mucosal surfaces (nose, throat). (Nagoya University)
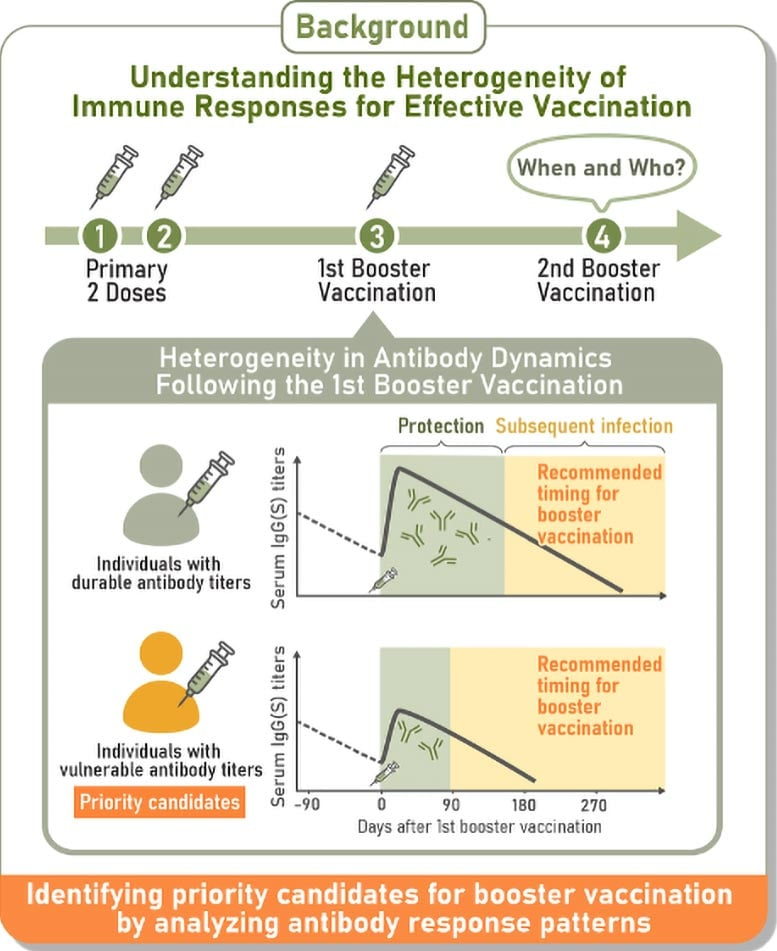
The Four Antibody Response Patterns
After the first booster, the data grouped people into four consistent trajectories of antibody behavior:
- Durable responders — those whose antibody levels stayed relatively high over time.
- Rapid-decliners — those whose antibodies rose strongly, but then dropped steeply.
- Vulnerable responders — those who never achieved very high antibody levels, and whose levels declined fast.
- Intermediate responders — those whose responses fell between the extremes.
Rough breakdown of proportions:
- Durable responders: ~ 29%
- Vulnerable responders: ~ 28%
- Rapid-decliners: ~ 19%
- The rest (~24%) were intermediate responders (Nagoya University)
Interestingly, the study’s abstract mostly emphasizes three groups (durable, vulnerable, rapid-decliner). The “intermediate” group is used in the press materials to cover people whose trajectories don’t clearly fall into the other three. (PubMed)
Infection Risk and Timing
What’s most important: which groups tended to have breakthrough infections—and when:
- The rapid-decliner group experienced infections sooner than the others, despite beginning with the strongest antibody peaks.
- A single snapshot IgG test (i.e. measuring antibody level at one point) could not detect which individuals were at higher risk. The predictive value only emerged when looking at how fast antibodies fell over months.
- Breakthrough rates were not wildly different in absolute terms: ~ 5.2% among durable responders, vs ~ 6% in vulnerable responders and rapid-decliners. But timing matters: rapid-decliners tended to have earlier attacks. (Nagoya University)
One more key observation: participants who got infected had lower levels of IgA (spike-specific IgA) in the weeks after vaccination than those who remained uninfected. Because blood IgA levels correlated well with nasal IgA, the authors suggest blood IgA could act as a surrogate marker for mucosal defense. (Nagoya University)
Why This Matters — Practical Takeaways
1. Peak antibody levels don’t tell the full story
Just because your antibody titer is high soon after vaccination doesn’t guarantee long protection. What matters is how steeply those levels decline.
2. Monitoring over time could guide boosters
If you belong to a rapid-decliner trajectory, you might benefit from earlier or more frequent booster doses.
Using blood IgA(S) levels as a biomarker could help identify people at elevated risk. But the feasibility (cost, accuracy, broad deployment) still needs investigation. (Nagoya University)
3. Biological causes still unknown
Why do some people decline quickly, while others maintain durable immunity? The authors point to possible influences like age, genetics, vaccine type, sleep, stress, medications, and more. But no definitive cause was established yet. (Nagoya University)
4. Generalizability and limitations
- This was a single-region cohort (Fukushima, Japan). Other populations with different demographics, variants, or vaccine schedules may behave differently.
- The absolute differences in infection rates were modest—the main insight is about timing and risk stratification, not huge gaps in infection frequency.
- It is observational, not interventional—the results show correlation, not definitive cause.
- Rolling out antibody-tracking or IgA screening broadly could be challenging in the real world, especially in low-resource settings.
Broader Context & Related Findings
To help put this study in context, here are a few related points:
- A recent long-term antibody kinetics study (over 3 years, ~8,000 samples) showed that after vaccination (or infection) there’s typically an initial rapid decline of antibodies, followed by a slower “plateau” phase where decay is much more gradual. (ScienceDirect)
- Other modeling work has already tried to predict antibody kinetics post-booster and to connect them to infection risk. (PMC)
- Past studies have documented that antibody titers (especially IgG) decrease over time after second and third vaccine doses if no infection occurs, consistent with what many expect. (MDPI)
- A complementary line of research suggests that the class switching of antibodies (e.g. towards IgG4) after repeated mRNA vaccination might correlate with higher risk of breakthrough infection. That raises subtle immunological questions about long-term antibody function. (Journal of Infection)
Putting all this together, the Nagoya University work stands out by offering a trajectory-based classification method and showing that the rate of decline matters.
What You Could Do
If you’re someone with access to antibody monitoring (for example, in a clinical or research setting), this study suggests:
- Don’t just measure your antibody level once; track it over time.
- Consider adding IgA(S) measurement (if feasible) to get a sense of your mucosal immune status.
- If you see steep decline, you might discuss earlier booster timing with your clinician (depending on guidelines and local policies).
For health systems and vaccine policy planners:
- Incorporate antibody decay modeling into booster scheduling instead of using one-size-fits-all timing.
- Investigate whether screening blood IgA post-vaccination is cost-effective and predictive.
- Conduct similar studies in diverse regions, age groups, and with newer variants to test the robustness of these response categories.
In summary: this paper offers a more refined lens to understand vaccine response—not just “do you have antibodies?” but “how fast do you lose them?” That insight helps explain why two people with strong early responses may eventually diverge in their protection levels. It opens a path toward personalized booster strategies and more precise immunity monitoring.
Reference: Longitudinal antibody titers measured after COVID-19 mRNA vaccination can identify individuals at risk for subsequent infection, Science Translational Medicine, DOI: 10.1126/scitranslmed.adv4214 (Science)





