MIT Researchers Develop a Selective Crystallization Method That Could Make Gene Therapy Drugs 10 Times Cheaper
Gene therapy is one of the most promising areas in modern medicine, offering the potential to treat — and sometimes cure — genetic diseases by delivering healthy genes into patients’ cells. But while the science behind it is revolutionary, the manufacturing process has been frustratingly inefficient and expensive. Researchers at the Massachusetts Institute of Technology (MIT) have now unveiled a new purification technique that could drastically cut production costs and speed up the manufacturing process.
The new approach, called selective crystallization, allows scientists to separate active, gene-carrying viral particles from inactive ones far more efficiently than the current standard methods. This innovation could make gene therapy treatments significantly more affordable and accessible to patients around the world.
The Manufacturing Problem Behind Expensive Gene Therapies
Most gene therapy drugs rely on adeno-associated viruses (AAVs) as delivery vehicles. These viruses act like small molecular packages, carrying a corrected or therapeutic gene into the patient’s cells. However, during manufacturing, the process often produces a large number of empty viral shells, known as capsids, which lack the therapeutic gene inside.
These empty capsids look nearly identical to the functional, or “full,” ones. They have the same protein sequence, nearly the same molecular weight, and a similar density. This similarity makes it extremely challenging to separate the two efficiently.
The result? Up to 90% of the viral particles produced during manufacturing are empty and therapeutically useless. Removing these unwanted particles is one of the most costly and time-consuming steps in gene therapy production.
In fact, purification alone can account for nearly 70% of the total manufacturing cost of a gene therapy drug. The inefficiency of existing purification methods has been a major bottleneck for scaling up production and reducing prices.
Why Current Purification Methods Fall Short
Most production systems today use a process called chromatography. In this method, the viral mixture is passed through a column filled with a specialized absorbent material. Because the physical and chemical differences between full and empty capsids are so small, the mixture has to go through multiple rounds of separation and filtration to reach acceptable purity levels.
This not only takes time — often 37 to 40 hours per batch — but also results in significant material loss, sometimes wasting 30–40% of the usable product. Even after all that effort, the end product typically contains about one-third inactive material.
These inefficiencies contribute to the astronomical cost of gene therapy treatments, some of which can reach millions of dollars per dose. The industry has long been searching for a faster, scalable, and more selective method of purification.
The Breakthrough: Selective Crystallization
The MIT research team, led by Vivekananda Bal, Richard Braatz, and colleagues from MIT’s Department of Chemical Engineering and Center for Biomedical Innovation, found inspiration in a technique used in the small-molecule pharmaceutical industry — preferential crystallization.
Crystallization is commonly used to purify small molecules, but it had never been applied to viral proteins or capsid-based biologics before. The researchers adapted the process for AAV purification and achieved remarkable results.
The key insight is that full and empty capsids have a tiny but measurable difference in their electrical charge. DNA molecules inside the full capsids carry a slight negative charge, while the capsid surface itself is positively charged. This subtle difference in charge density affects how these particles behave during crystallization.
Under the right conditions — involving precise combinations of salt, precipitant, and pH — the full capsids crystallize out of the solution, while the empty ones remain in suspension.
This single-step process not only separates full from empty capsids but also removes other impurities like cell debris, without requiring multiple pre-processing and post-processing stages.
Results That Could Transform Biomanufacturing
According to the MIT researchers, the crystallization process takes only about four hours, compared to nearly 40 hours for chromatography. That’s roughly 10 times faster.
The results are equally impressive:
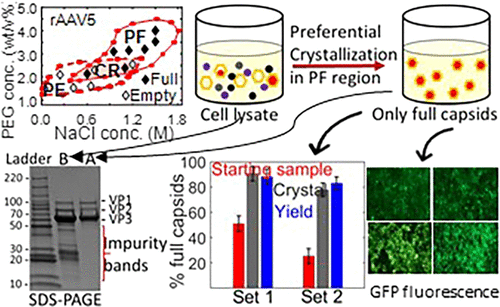
- Full capsid enrichment: Over 80% purity in just one crystallization step.
- Yield: Above 90%, meaning minimal loss of valuable product.
- Scalability: The process can be easily adapted for large-scale pharmaceutical production.
The team has already filed for a patent through the MIT Technology Licensing Office and is in talks with several pharmaceutical companies to begin pilot trials. Depending on industrial validation, the method could be commercialized within the next few years.
The researchers estimate that this technique could reduce the cost of gene therapy manufacturing by five to ten times, a potential game-changer for the field.
Technical Specifics of the Method
The team tested their approach on multiple AAV serotypes, including rAAV5, rAAV8, and rAAV9, which are commonly used in clinical gene therapy trials. They created phase diagrams to map out the specific conditions that favor crystallization of full capsids while keeping empty ones soluble.
For example, one patented method uses polyethylene glycol (PEG) as a precipitant (at 0.5–8% w/v), sodium chloride as a salt (0.01–2.5 M), and a pH range between 5.5 and 7.5. Under these conditions, full capsids form solid crystals that can later be re-dissolved into pharmaceutical formulations.
The crystals are stable and can potentially be stored long-term at −10 to −30°C with appropriate cryoprotectants, or short-term at 2–20°C. This opens up the possibility of new storage and transport advantages, as crystalline forms of viral vectors could be more robust than liquid formulations.
Why This Discovery Matters
The implications of this breakthrough go far beyond academic curiosity. The global gene therapy market is projected to grow rapidly, but its success depends heavily on improving manufacturing efficiency. If the purification process becomes faster and cheaper, therapies that currently cost millions could become affordable to many more patients.
The method could also reduce immune-related side effects, since fewer empty capsids would mean a smaller risk of triggering unwanted immune responses.
Moreover, the streamlined process could encourage smaller biotech companies to enter the gene therapy market, as the reduced cost and complexity lower the barrier to entry.
This innovation could also influence how other biologic drugs — such as viral vaccines, mRNA carriers, or nanoparticle-based treatments — are purified in the future.
The Road Ahead
While the results are promising, several challenges remain before the technique can become industry standard. Large-scale production will need to prove that crystallization can maintain high yield and purity across big batches, all under cGMP (current Good Manufacturing Practice) conditions.
Regulatory agencies will also need to evaluate how the crystalline viral particles behave during formulation, storage, and administration. The team’s early results show potential, but real-world pharmaceutical validation takes time.
Nonetheless, the enthusiasm from biotech companies suggests that the field sees real potential. The MIT researchers are already collaborating with industrial partners, who are testing their own gene therapy samples using this crystallization technique.
If successful, this could represent a major shift in how gene therapies are produced, bringing us one step closer to affordable, scalable cures for a wide range of genetic diseases.
A Quick Background on Gene Therapy and AAVs
Gene therapy works by inserting a healthy copy of a gene into cells to replace or correct a faulty one. The most common way to deliver these genes is through viral vectors, modified viruses that can safely carry therapeutic DNA.
Among these, adeno-associated virus (AAV) has become the most widely used vector, accounting for about 60% of all gene therapy trials since 2017. AAVs are preferred because they are non-pathogenic, have a stable structure, and can target a wide range of tissues.
However, the challenge of producing pure, gene-carrying AAV particles has slowed progress and inflated costs. That’s why MIT’s new crystallization-based approach could play such a vital role in the field’s evolution.
Research Reference:
Selective Enrichment of Full Capsids of Adeno-Associated Virus – ACS Nano (2025)