New Study Reveals PFAS Are Much More Acidic Than Previously Believed
Scientists have taken a big step forward in understanding per- and polyfluoroalkyl substances (PFAS), the so-called “forever chemicals.” A new study led by the University at Buffalo shows that many PFAS are far more acidic than earlier research suggested. This finding matters because acidity affects how these chemicals move through the environment, persist in water, and potentially impact human health.
The Importance of Measuring Acidity
PFAS are made up of a highly fluorinated, water-repelling tail and a more water-attracting headgroup. The headgroup is often strongly acidic, which means it readily sheds a proton and takes on a negative charge. When that happens, the compound dissolves easily in water and becomes more mobile. This mobility is why PFAS are so difficult to remove from ecosystems and why they spread so far once released.
The acidity of a chemical is measured through its pKa value. A lower pKa means the substance is more acidic and more likely to exist in its negatively charged form. Until now, there has been major disagreement in the scientific community about the true pKa values of PFAS compounds. Different teams using older methods reported inconsistent numbers, sometimes off by several orders of magnitude.
Why Previous Measurements Were Flawed
Traditional methods of measuring acidity often produced unreliable data for PFAS. One major issue is that PFAS molecules like to stick to glass surfaces, which disrupts experiments that rely on measuring bulk concentrations in solution. Another problem is the heavy use of organic solvents to get PFAS into solution, which also biases results.
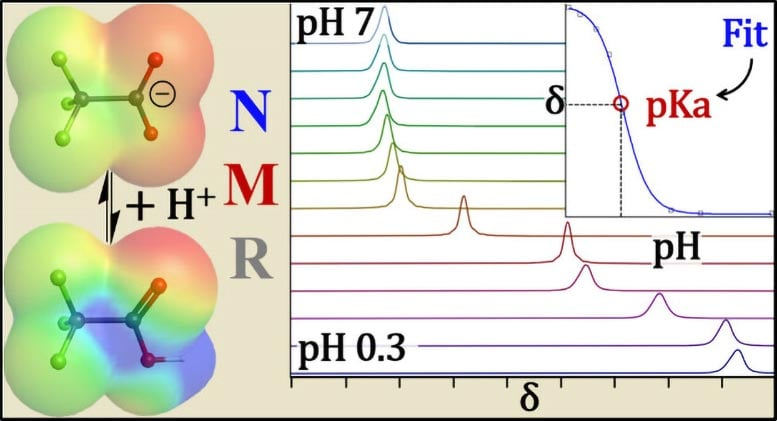
Because of these issues, pKa values for key PFAS compounds such as perfluorooctanoic acid (PFOA) have varied widely in the past, leaving regulators and researchers without a clear picture.
A New NMR-Based Approach
To fix this, the UB team applied nuclear magnetic resonance (NMR) spectroscopy — specifically fluorine and proton NMR. This technique acts like an MRI for molecules, allowing researchers to directly observe how atoms respond in charged versus neutral states.
When a PFAS headgroup becomes negatively charged, nearby fluorine atoms shift in response to radio waves at different frequencies. By analyzing these shifts, the researchers could determine whether the molecule was charged or not — eliminating the experimental biases that plagued earlier methods.
For the most acidic PFAS (those with pKa values below zero), it’s not possible to generate neutral forms in standard lab conditions. To handle this, the researchers paired NMR experiments with density functional theory (DFT) calculations, predicting the expected behavior of both neutral and ionized forms. This hybrid approach gave them confidence in their results and allowed them to report values with more accuracy than ever before.
The Numbers: How Acidic Are PFAS Really?
The team measured the acidity of 10 PFAS compounds and three common breakdown products. The results were consistently lower than previously reported values, meaning the chemicals are more acidic than thought. Some key highlights:
- PFOA (Perfluorooctanoic acid): New measurement is –0.27. Older experimental studies reported values as high as 3.8 and more commonly around 1. Computational methods previously estimated around 0.24–0.34. This new number means PFOA exists in its negatively charged form under virtually all environmental conditions.
- GenX (replacement for PFOA in Teflon manufacturing): Found to be about 1,000 times more acidic than previously reported.
- TFA (Trifluoroacetic acid): Newly measured at 0.03, compared to earlier estimates of 0.30–1.1. This chemical is increasingly detected worldwide and is likely carried through the atmosphere before being deposited by rain.
- MFA (Monofluoroacetic acid): pKa measured at 2.58 ± 0.03.
- DFA (Difluoroacetic acid): pKa measured at 1.22 ± 0.03.
The team also determined pKa values for emerging PFAS that had never been measured before, including 5:3 fluorotelomer carboxylic acid (5:3 FTCA) and ether-based PFAS such as NFDHA and PFMPA. These newer PFAS are of growing concern because of their potential health impacts and regulatory challenges.
Why This Matters for the Environment and Health
Knowing the true acidity of PFAS changes how scientists model their environmental behavior. Whether a PFAS molecule dissolves in water, binds to soil or membranes, or potentially volatilizes into the air depends on its pKa. More accurate values mean researchers can better predict how PFAS move and where they will end up.
This work also helps with remediation strategies. If regulators and engineers know which PFAS are more mobile and persistent, they can design better methods for cleanup. Additionally, the new data provides a benchmark for machine learning models that predict the properties of newly discovered PFAS when experimental reference standards aren’t available.
The Bigger Picture
This study clears up years of uncertainty about PFAS acidity. By showing that PFAS are generally more acidic than thought, it emphasizes just how mobile and persistent these chemicals are. For regulators, policymakers, and scientists, this is critical information for tracking contamination and designing responses.
The research was supported by the U.S. National Science Foundation and carried out in collaboration with St. Bonaventure University and Spain’s Institute of Environmental Assessment and Water Research.
TL;DR:
A University at Buffalo–led study shows PFAS are far more acidic than previously believed, with new NMR-based methods giving accurate pKa values. Results reveal stronger acidity for PFOA, GenX, TFA, and emerging PFAS, reshaping understanding of their mobility and persistence.
Reference: Experimental Determination of pKa for 10 PFAS, Mono-, Di-, and Trifluoroacetic Acid by 19F-NMR





