Red Light and Recyclable Catalysts: A Greener Path for Photocatalysis
Modern chemistry is steadily shifting toward sustainable, low-waste, and energy-efficient processes, and a team at the Center for Research in Biological Chemistry and Molecular Materials (CiQUS) in Spain has just made an important stride in that direction.
Their recent study, published in the Journal of the American Chemical Society (JACS), describes a new red-light-driven photocatalytic method that uses recyclable solid catalysts to perform chemical reactions cleanly and efficiently. The research, led by Martín Fañanás-Mastral and Manuel Souto Salom, introduces a practical way to use low-energy red light and covalent organic frameworks (COFs) to promote sustainable reactions — a notable step toward greener organic synthesis.
Why This Research Matters
Traditional photocatalysis — using light to drive chemical reactions — often depends on homogeneous catalysts, which dissolve in the reaction medium. These are effective but wasteful, as they can’t be recovered or reused easily. On top of that, most of these systems require blue or ultraviolet (UV) light, which consumes more energy and has limited penetration in dense mixtures. This means they’re not ideal for large-scale or biological applications.
The new approach by CiQUS researchers solves both problems at once: it uses heterogeneous (solid) catalysts that are fully recyclable and are activated by red light, which is gentler, less energy-intensive, and penetrates deeper into reaction media. The result is a cleaner, more efficient, and more sustainable form of photocatalysis that could have applications far beyond the lab.
Understanding Covalent Organic Frameworks (COFs)
At the core of this innovation are Covalent Organic Frameworks, or COFs. These are porous, crystalline materials built from organic molecular units connected in precise geometric patterns — a bit like molecular LEGO. Unlike Metal-Organic Frameworks (MOFs), which use metal nodes, COFs are entirely organic, making them lighter, more tunable, and often more stable under light and heat.
Because of their large surface area, orderly pores, and design flexibility, COFs are ideal for capturing and transferring light energy. They can be fine-tuned to absorb specific wavelengths, which makes them especially interesting for photocatalysis, where light absorption determines how effectively a reaction proceeds.
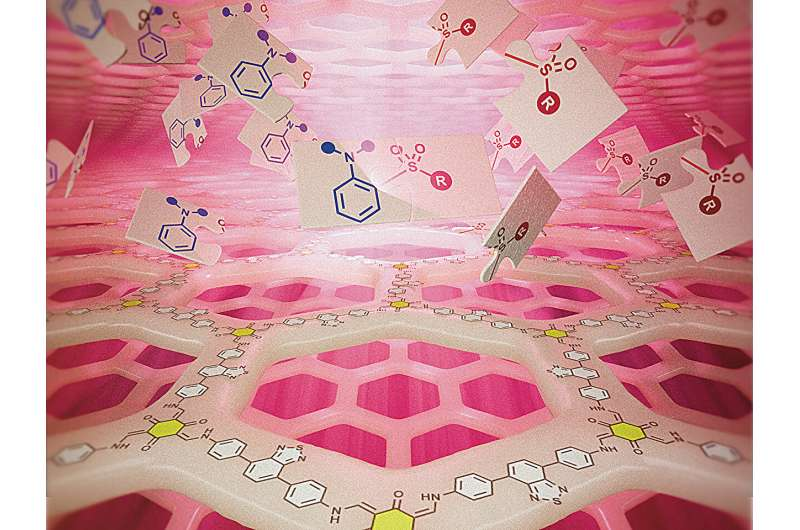
In this case, the CiQUS team designed a COF that includes a benzothiadiazole-based photoactive unit. This molecular fragment absorbs red light efficiently, creating reactive species that drive chemical transformations.
The Core Reaction: Red-Light-Driven Sulfonylation
To showcase their system’s power, the researchers used it for a C(sp²)–H sulfonylation of anilines. This reaction produces sulfones, a class of compounds with major industrial and pharmaceutical importance. Sulfones appear in many drugs, agrochemicals, and biologically active molecules because they enhance molecular stability, solubility, and biological compatibility.
Traditionally, sulfone synthesis often requires harsh oxidants, high temperatures, or complex catalysts, leading to waste and energy consumption. The new COF-based system simplifies the process dramatically.
Here’s how it works in essence:
- The benzothiadiazole unit in the COF absorbs red light.
- The absorbed light energy creates reactive intermediates — likely sulfonyl radicals from sulfinate salts.
- These radicals then react directly with aniline derivatives, forming C–S bonds and yielding sulfones.
The reaction occurs under mild conditions, with minimal catalyst loading, and the COF catalyst can be recovered and reused at least six times without losing performance. That’s a major step toward practical, recyclable photocatalysts that reduce both cost and waste.
Advantages of Red Light in Chemistry
Most photocatalytic systems rely on blue or UV light, which has high energy but several drawbacks. UV light can damage sensitive organic molecules and often causes unwanted side reactions. Blue light, though safer, still demands more energy and doesn’t penetrate reaction mixtures deeply — making it inefficient for larger or opaque systems.
Red light, on the other hand, has several benefits:
- Lower energy consumption, meaning it’s gentler on both the molecules and the environment.
- Deeper penetration, which helps ensure uniform reaction in thicker or denser media.
- Compatibility with biological systems, since red light is less damaging to cells and tissues.
These qualities make red light particularly interesting for biomedicine, photoresponsive materials, and large-scale photoreactors where efficient light distribution is key.
Reusability and Efficiency
The recyclability of the catalyst is one of the study’s most impressive results. Because the COF is a solid, it can be easily separated from the reaction mixture — usually by filtration — and reused. The researchers reported at least six cycles of reuse without loss of activity or structural degradation.
That kind of durability is critical for making photocatalysis viable on an industrial scale. In conventional systems using homogeneous catalysts, the loss of catalyst after each reaction represents both a cost and environmental burden.
Moreover, the researchers found that the reaction worked across a broad substrate scope, meaning that many different aniline derivatives reacted efficiently. This generality is an essential feature for any practical synthetic method.
The Role of Internal Collaboration
The study also highlights the synergy between different research groups within CiQUS. One group brought deep expertise in organic synthesis and photocatalysis, while the other focused on materials design and COF synthesis. Their collaboration was supported by the CiQUS-Synergy program, which aims to encourage cross-disciplinary projects within the center.
Such collaboration is crucial in modern chemistry, where solving sustainability challenges often requires combining materials science, physical chemistry, and synthetic strategy.
The Bigger Picture: Toward Sustainable Photochemistry
This research sits at the intersection of several major scientific trends:
- Green chemistry, which emphasizes minimizing waste and energy use.
- Heterogeneous catalysis, which enables recycling and scalability.
- Photochemistry, which uses light — a renewable energy source — instead of heat or harsh reagents.
The combination of red light and COFs could revolutionize how photocatalytic processes are designed. Instead of relying on fragile molecular dyes or precious metal complexes, chemists can now use robust, modular organic materials.
The potential applications extend far beyond sulfonylation reactions. Red-light-active COFs could be used for photooxidation, reduction, or coupling reactions, solar energy conversion, or even light-controlled drug release systems. Since red light is safer for biological matter, these materials could find use in photomedical technologies, such as photodynamic therapy or light-triggered biochemical synthesis.
What Are COFs and Why Are They Special?
Since this study centers on COFs, it’s worth digging a little deeper into what makes them unique. COFs are relatively new materials — first reported in the mid-2000s — and they have quickly become one of the hottest topics in materials chemistry. They are built from light elements like carbon, nitrogen, oxygen, and boron, connected by strong covalent bonds to form rigid frameworks.
Some standout features of COFs include:
- Crystallinity and porosity: Their ordered structure provides large internal surface areas (hundreds to thousands of square meters per gram).
- Design flexibility: Chemists can tailor the framework’s building blocks to control light absorption, pore size, or electronic behavior.
- Stability: Unlike many polymers or molecular dyes, COFs are stable under heat, solvents, and light exposure.
- Heterogeneous operation: They don’t dissolve, allowing easy recovery and reuse.
In photocatalysis, these properties mean that COFs can serve as light-harvesting solids, capturing photons and transferring that energy to drive chemical transformations.
Limitations and Future Directions
While the results are exciting, there’s still room for improvement. The researchers demonstrated six reuse cycles, but long-term durability (tens of cycles) will need further testing. Also, scale-up challenges remain — light penetration, mass transfer, and reactor design all influence performance on industrial scales.
Another question is cost: although COFs are made from organic materials, their synthesis can still involve complex steps. Simplifying production and improving yield will be important for widespread adoption.
Nonetheless, the principles shown in this study — combining recyclable materials with low-energy light — point clearly toward a sustainable future for photocatalysis.
Reference
Research Paper: Red-Light-Driven C(sp²)–H Sulfonylation of Anilines Using a Recyclable Benzothiadiazole-Based Covalent Organic Framework
Published in: Journal of the American Chemical Society (JACS), 2025
Authors: Saúl Alberca, Martín Fañanás-Mastral, Manuel Souto Salom, and collaborators at CiQUS