Scientists Identify Biological Basis of Long COVID Brain Fog

One of the most troubling and disruptive symptoms of Long COVID is what many patients describe as “brain fog.” This term covers a range of issues such as poor memory, difficulty concentrating, slower processing speed, and trouble with everyday tasks that require focus.
For more than four years since the beginning of the COVID-19 pandemic, researchers have been trying to find clear biological explanations for these cognitive problems. Now, a team of scientists from Yokohama City University in Japan has discovered a potential molecular mechanism that may finally explain what is happening in the brains of people struggling with Long COVID.
The study, published in Brain Communications on October 1, 2025, highlights changes in specific brain receptors that could serve as both a biomarker and a therapeutic target. This represents one of the most important steps so far toward diagnosing and treating the cognitive complications of Long COVID.
What the Researchers Did
The Japanese research team, led by Professor Takuya Takahashi at the Graduate School of Medicine at Yokohama City University, focused on molecules called AMPA receptors (AMPARs). These are proteins found in nerve cells that play a critical role in synaptic transmission, learning, and memory. Abnormalities in AMPARs have previously been linked to other brain disorders such as schizophrenia, bipolar disorder, depression, and dementia.
Because AMPARs are so central to cognitive function, the scientists wondered if disruptions in these receptors might also explain the brain fog experienced by Long COVID patients. Until now, researchers have struggled to get molecular-level data from the human brain because it is extremely difficult to measure these receptors directly in living patients.
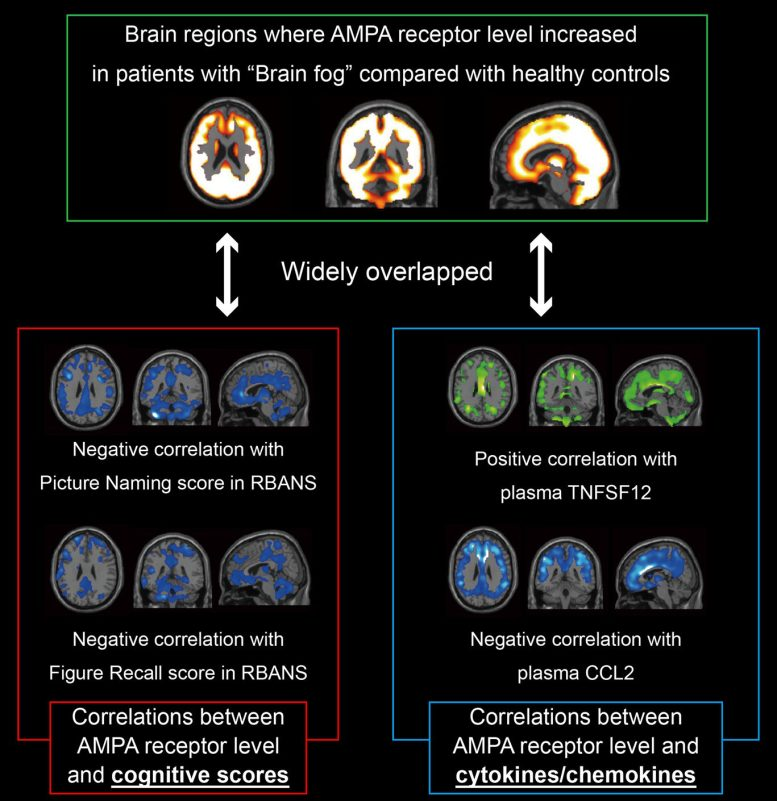
To overcome this challenge, the team used a new brain imaging technique known as [11C]K-2 AMPAR PET imaging. This allowed them to visualize and measure AMPAR density across the brain in real time.
Study Participants and Imaging Results
The study compared 30 patients with Long COVID and brain fog symptoms against 80 healthy individuals. The imaging results were striking.
- The Long COVID group showed a widespread increase in AMPAR density across multiple brain regions.
- The higher the density of AMPARs, the worse the cognitive impairment reported by the patients.
- Levels of inflammatory biomarkers in blood samples also correlated with the elevated AMPAR density. This suggests a direct link between systemic inflammation and changes in brain receptor function.
The team’s analysis went even further. Using the PET imaging data, they were able to distinguish Long COVID patients from healthy controls with 100% sensitivity and 91% specificity. In other words, the imaging results alone were enough to identify which participants had Long COVID brain fog with an impressive degree of accuracy.
Why This Finding Matters
The discovery of increased AMPAR density as a molecular marker has major implications:
- It provides a biological explanation for brain fog in Long COVID patients.
- It opens up the possibility of using AMPAR PET imaging as a diagnostic tool.
- It points toward a new therapeutic strategy. Drugs that suppress or regulate AMPAR activity could potentially reduce cognitive problems without disrupting normal brain function, though much more research will be needed to ensure safety.
This is the first time that scientists have been able to directly tie a measurable molecular change in the brain to the cognitive symptoms experienced by Long COVID patients.
The Bigger Picture: What We Already Knew About Brain Fog
Before this study, several other theories were proposed to explain brain fog:
- Neuroinflammation: Long-lasting immune activation in the brain.
- Blood-brain barrier leaks: Damage allowing harmful molecules to seep into brain tissue.
- Vascular damage and microclots: Small clots and vessel injury affecting blood flow to brain areas.
- Hypoxia and oxidative stress: Oxygen deprivation and cell damage caused by the initial infection.
- Autoimmunity or viral reactivation: The immune system mistakenly attacking the body, or dormant viruses like Epstein-Barr reactivating.
Previous brain scans (using FDG-PET or MRI) sometimes showed reduced metabolism in certain brain regions of Long COVID patients, but these results could not explain the underlying molecular mechanisms. This new AMPAR study directly connects molecular changes to patient symptoms in a way earlier research could not.
Funding and Support
This research was made possible through a mix of academic and community support:
- Funding from the Takeda Science Foundation.
- Grants from the Japan Agency for Medical Research and Development (AMED), including grant JP24wm0625304.
- Support from the Japan Science and Technology Agency (JST) fellowship program (grant JPMJFS2140).
- Donations via the READYFOR crowdfunding platform.
The combination of institutional backing and community fundraising shows how urgent the need for answers about Long COVID remains.
Other Related Research
While this Japanese study is the first to identify AMPAR overexpression as a biomarker, other groups around the world are also pushing forward with related investigations:
- UC Davis and UCSF researchers are using a total-body PET scanner (uEXPLORER) with tracers that tag activated T-cells. This could help map immune activation across the entire body of Long COVID patients, including the brain.
- Other studies have found persistent neuroinflammation in Long COVID patients even two years after infection, using different types of PET imaging.
- Research from 2024 suggested that blood-brain barrier leaks may contribute to brain fog. Higher levels of a brain protein called S100β were found in blood samples, which normally should not be present outside the brain. MRI scans confirmed leakage in some patients.
- EEG and metabolic studies have shown slowed brain activity and hypometabolism in frontal and temporal lobes of affected individuals.
Taken together, all of these findings support the idea that Long COVID brain fog is a real, measurable clinical condition rooted in brain biology—not just a vague, subjective complaint.
Limitations of the Study
Although the AMPAR findings are groundbreaking, the study is still early-stage research and comes with limitations:
- Sample size: Only 30 Long COVID patients were examined. A larger, more diverse cohort will be needed to confirm results.
- Cross-sectional design: This study measured patients at one point in time. Longitudinal studies will be required to track how AMPAR density changes over time or in response to treatment.
- Specificity: It’s not yet clear if increased AMPAR density is unique to Long COVID or if it could appear in other neurological conditions.
- Therapeutic risks: AMPARs are essential for normal memory and learning. Broadly suppressing them with drugs could have side effects, so any potential treatment would need to be very carefully developed.
- Practical challenges: PET imaging with specialized tracers is expensive and not widely available. Using it as a large-scale diagnostic tool may not be feasible in many healthcare settings.
What This Could Mean for the Future
The implications of this discovery go beyond just understanding Long COVID:
- Diagnostic use: If confirmed, AMPAR PET imaging could provide the first reliable biomarker for diagnosing brain fog.
- Therapeutic development: Drug companies may begin looking at compounds that can safely regulate AMPAR activity.
- Patient stratification: Doctors may one day be able to determine which Long COVID patients are most likely to benefit from AMPAR-targeted treatments.
- Broader neuroscience impact: Because AMPAR dysfunction is also linked to psychiatric and neurodegenerative conditions, this research could have ripple effects far beyond COVID-related illness.
Learning More About AMPA Receptors
Since AMPARs are at the center of this discovery, it’s worth understanding them better.
- Function: AMPA receptors are glutamate receptors, meaning they respond to glutamate, the brain’s most common excitatory neurotransmitter. They control the flow of ions into neurons, which allows cells to send signals.
- Role in memory: AMPARs are central to a process called synaptic plasticity, which underlies learning and memory. Increasing or decreasing the number of AMPARs at synapses changes how strong connections between neurons become.
- Clinical importance: Abnormal AMPAR activity has been tied to Alzheimer’s disease, epilepsy, schizophrenia, and other neurological conditions. Drugs that target these receptors already exist for other disorders, though none are currently approved specifically for Long COVID.
Conclusion
The Japanese team’s discovery that Long COVID brain fog is linked to a systemic increase in AMPA receptors is a major step forward in understanding the condition. It provides the clearest biological evidence yet that cognitive problems after COVID-19 are not just psychological or subjective, but grounded in real molecular changes in the brain.
While more research will be needed to confirm and expand upon these results, the findings represent hope for the millions worldwide who continue to live with the long-term consequences of COVID-19.





