Smart Flow Cytometer Turns Clogging Into a Useful Tool
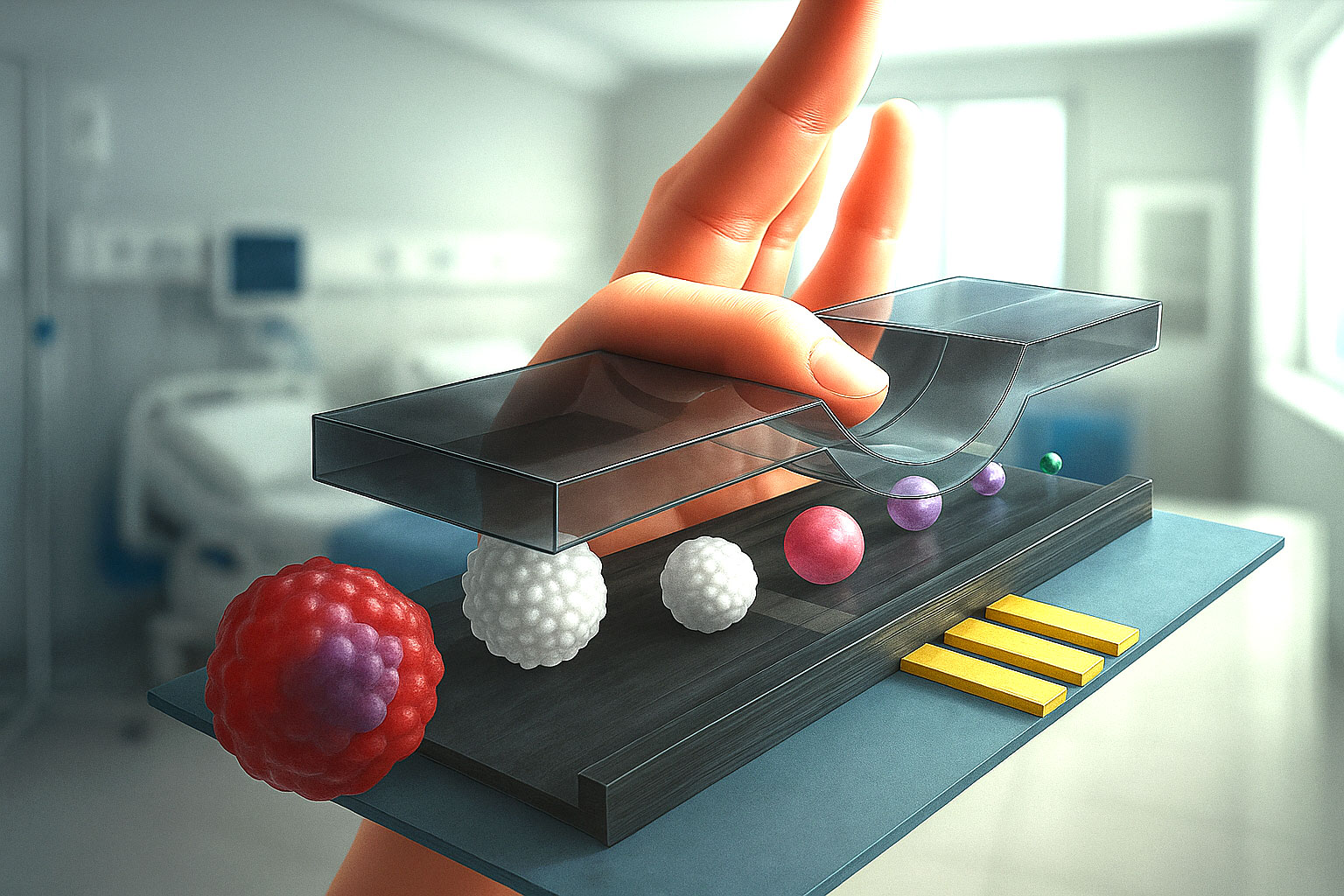
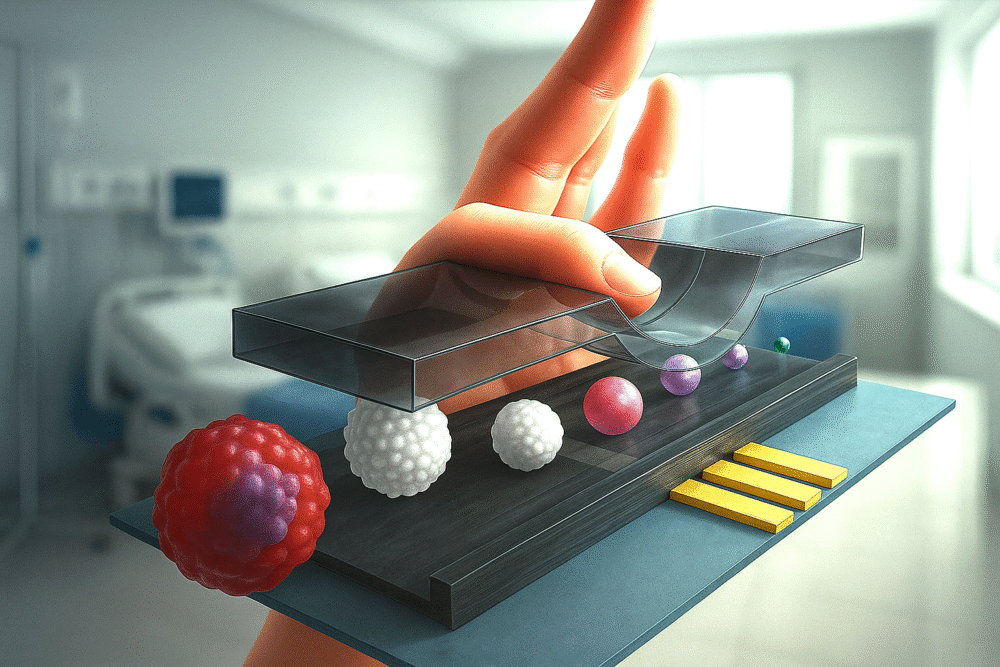
Flow cytometry has been one of the most important technologies in biomedical research for decades, allowing scientists to study individual cells with great detail. Traditionally, this method uses lasers and fluorescent tags to identify and analyze cells. But a team of researchers from the Nara Institute of Science and Technology (NAIST) in Japan has developed something new: an adaptive impedance flow cytometer that not only improves sensitivity but also cleverly uses the problem of clogging to its advantage.
This breakthrough was recently published in the journal Lab on a Chip under the title “A long-term universal impedance flow cytometry platform empowered by adaptive channel height and real-time clogging-release strategy.” The work was led by Associate Professor Yaxiaer Yalikun, together with Trisna Julian, Tao Tang, Naomi Tanga, Yang Yang, and Professor Yoichiroh Hosokawa.
Why Flow Cytometry Matters
Flow cytometry is a cornerstone of modern medicine and life sciences. It works by passing cells through a narrow microfluidic channel, then analyzing them one by one as they pass through a laser beam. With fluorescence tags attached to cells, researchers can detect and measure various properties such as size, structure, or the presence of specific proteins. This technology has played a huge role in drug discovery, immunology, cancer research, and diagnostics.
But there’s a downside: fluorescence tagging can be expensive, time-consuming, and sometimes not suitable for certain applications. This limitation is where impedance flow cytometry (IFC) comes in as a powerful alternative.
What Is Impedance Flow Cytometry?
Instead of lasers and fluorescence, impedance flow cytometry uses electrodes to measure changes in electrical impedance as cells or particles move through a channel. In simpler terms, when a particle flows past the electrodes, it disrupts the current in a measurable way. This allows researchers to identify and characterize cells without chemical tags.
The benefits are clear: it’s label-free, less costly, and less preparation-intensive. However, impedance flow cytometry has its own challenges. The biggest one comes from the distance between the cell and the electrodes. If a cell flows closer to one electrode, the signal is stronger. If it’s farther away, the signal weakens. This variation leads to inconsistencies in sensitivity and accuracy.
The height of the microfluidic channel also plays a role. A channel that is too tall creates space for cells to drift away from the electrodes, while one that is too small risks clogging. Balancing this has been a major obstacle — until now.
The NAIST Solution: A Dynamic Microfluidic Channel
The Japanese research team designed a system where the height of the microfluidic channel can be dynamically adjusted in real time.
Here’s how they did it:
- They used a thin metal probe mounted on the vertical axis of a precise XYZ translation stage.
- This probe pressed gently against the top of a 30-micrometer-high microchannel, slightly compressing it.
- By lowering or raising the probe, the team could squeeze the channel to reduce its height or relax it to restore the original size.
By squeezing the channel, cells were forced to flow closer to the electrodes, which significantly improved signal strength and reduced measurement variability.
Key Results From the Experiments
Through both simulations and lab experiments, the researchers found that:
- Reducing the channel height by one-third led to a 3.2-fold increase in signal strength.
- Signal variability was reduced by about half, improving precision.
- The system could easily distinguish between cells and particles of different sizes, something that’s crucial for real-world biomedical analysis.
To validate the approach, the team tested it with a mix of 6-micrometer beads and yeast cells, and later with cell lines such as A549, C6, and NIH/3T3. In all cases, the adaptive channel improved detection and discrimination compared to conventional setups.
Turning Clogging Into an Advantage
Clogging is usually the bane of microfluidics. When particles accumulate and block the channel, it stops the system and requires manual cleaning. But instead of fighting against this, the NAIST team came up with a clever strategy: controlled clogging.
They integrated a camera system with an object detection algorithm to monitor the flow in real time. The software allowed them to deliberately squeeze the channel to the point where clogging was about to happen — maximizing sensitivity in the process — and then release the deformation just before an actual blockage occurred.
This way, clogging became part of the system’s design, acting as a natural feedback mechanism. The result is a clog-resistant, self-adjusting flow cytometer that can run stably for longer periods.
Why This Matters for Medicine and Research
This development is more than a clever engineering trick. It has real implications:
- More sensitive diagnostics: With improved accuracy, this platform could detect subtle cellular differences important for early disease detection.
- Point-of-care testing: The low-cost and label-free nature of this method means it could be adapted for small, portable diagnostic devices.
- Drug development: Pharmaceutical labs could use it for high-throughput testing, especially where quick, consistent single-cell analysis is needed.
- Biomedical research: Because the system can handle different cell types by simply adjusting the channel, it’s versatile for many applications.
Extra Knowledge: Microfluidics and Why Clogging Happens
To better understand this breakthrough, it helps to look at microfluidics itself. Microfluidics is the science of controlling fluids in extremely tiny channels, often thinner than a human hair. It has been applied in fields ranging from lab-on-a-chip diagnostics to inkjet printing.
Clogging in microfluidics is common because:
- Particles can stick to the channel walls.
- Cell suspensions are rarely uniform, so clumps of cells form.
- Any variation in channel size, even at the micrometer scale, can trap particles.
In most systems, clogging is a disaster. It halts experiments and introduces errors. The NAIST team’s approach to embrace and control clogging rather than avoiding it is a shift in thinking that could inspire other microfluidic technologies.
Impedance Flow Cytometry in Broader Context
Impedance flow cytometry isn’t new, but it has always been a bit overshadowed by fluorescence-based methods. However, it’s starting to gain ground for several reasons:
- It doesn’t rely on costly reagents.
- It can handle samples where labeling isn’t possible or desirable.
- It can be faster and more automated.
The NAIST innovation addresses the two big problems holding IFC back: sensitivity and stability. By making the channel height adaptable and adding a smart anti-clogging strategy, this research paves the way for a universal, high-performance platform that could finally bring IFC into mainstream biomedical use.
Future Prospects
There are several promising directions this research could take:
- Commercial devices: With industry partnerships, this design could be developed into ready-to-use diagnostic tools.
- Broader sample types: Beyond mammalian cells and yeast, the platform could be tested with bacteria, parasites, or even environmental particles.
- Integration with AI: The current object detection algorithm could be expanded with machine learning to optimize channel adjustments automatically.
- Other microfluidic applications: Similar adaptive channel strategies could be applied in filtration, droplet microfluidics, or long-term monitoring systems where clogging is a major challenge.
Wrapping Up
What the NAIST team has done is both practical and elegant. By introducing a dynamically adjustable channel height and using clogging as part of the design, they’ve significantly advanced impedance flow cytometry. This research not only boosts sensitivity and consistency but also opens up new possibilities for medical diagnostics, research, and drug development.
This could mark a turning point where impedance flow cytometry steps out of the shadow of fluorescence-based methods and becomes a mainstream tool for cell analysis.
Research Reference: A long-term universal impedance flow cytometry platform empowered by adaptive channel height and real-time clogging-release strategy