Turning Waste Plastics into High-Performance Carbon Nanomaterials
Scientists have found a way to give our discarded plastics a powerful new purpose — transforming them into high-performance carbon nanomaterials that can help clean water, power batteries, and drive sustainable energy technologies. This research, led by a team from the University of Adelaide in collaboration with the Australian Nuclear Science and Technology Organisation (ANSTO), was recently published in Nature Communications (2025). It demonstrates that what we once viewed purely as waste can, in fact, become an advanced resource for the future of green technology.
The Plastic Problem We All Know
Plastics have been both a blessing and a curse for modern life. They are durable, cheap, and versatile, but their resilience is also what makes them such a nightmare for the environment. Each year, millions of tons of plastics — from bottles, packaging, and everyday products — end up in landfills or oceans. Their chemical stability means they take centuries to degrade, while traditional recycling methods often struggle to handle the wide mix of polymers in our waste stream.
Conventional recycling typically downcycles plastic into lower-quality products. It’s inefficient, limited in scope, and can’t keep pace with global plastic production. The University of Adelaide team wanted to change that — to upcycle plastics into materials that are not just useful, but highly valuable in advanced technologies.
From Plastic Trash to Atom-Level Precision Materials
The researchers developed a universal and scalable method that converts common plastics — including PET, PVC, polyethylene (PE), polypropylene (PP), and their mixtures — into something called single-atom catalysts (SACs).
SACs are cutting-edge materials in which individual metal atoms are anchored and isolated on a carbon substrate, such as graphene. These single atoms serve as highly efficient catalytic sites, dramatically improving performance in chemical reactions. Because every atom is exposed and active, SACs use metal resources with maximum efficiency — making them both powerful and cost-effective.
Here’s the big breakthrough: instead of requiring pure and uniform starting materials, this new method works across different kinds and mixtures of plastics, producing gram-scale yields that indicate it’s ready for real-world applications. That’s rare in materials science, where lab-scale discoveries often struggle to scale up.
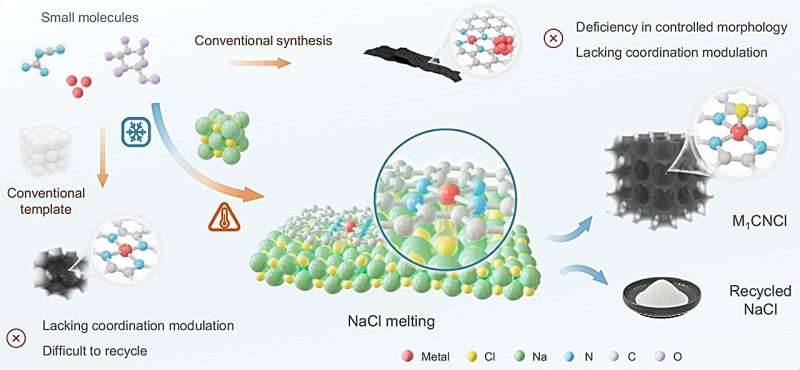
How It Works: The Salt-Templated Transformation
At the heart of this innovation is a salt-templating process using sodium chloride (NaCl) — yes, ordinary table salt — as a template and reaction medium. The researchers call the resulting catalysts “M₁CNCl,” where M represents a metal such as iron (Fe), nickel (Ni), or cobalt (Co).
The process unfolds as follows:
- Mixing Ingredients:
Metal salts (like FeCl₂·4H₂O), a nitrogen source (dicyandiamide), a carbon source (glucose), and NaCl are dissolved together with the shredded plastic feedstock. This mixture forms a homogeneous precursor solution. - Freeze-Drying:
The mixture is freeze-dried to form solid cubes of salt containing the embedded metal and carbon precursors. - High-Temperature Pyrolysis:
The cubes are then heated to around 900°C in an inert atmosphere. At this temperature, NaCl melts and serves as a molten template, guiding the formation of a porous, carbon-rich structure derived from the decomposed plastics. - Template Removal:
After heating, the salt is washed away, leaving behind a porous carbon framework with metal atoms anchored as single atoms within the carbon matrix.
This structure — metal atoms coordinated by nitrogen and chlorine atoms — creates a stable, uniform catalytic environment. The use of NaCl is particularly clever: it’s cheap, easy to handle, and can be recovered at a rate of about 90% for reuse.
The Role of Synchrotron X-Ray Characterization
To confirm that the metals were indeed present as single atoms (and not forming unwanted nanoparticles), the team used X-ray Absorption Spectroscopy (XAS) at ANSTO’s Australian Synchrotron in Melbourne. This powerful technique can probe the atomic-scale structure of materials, distinguishing isolated atoms from clusters or particles.
The XAS data, along with high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM), verified that the metals were atomically dispersed and chemically bound within the carbon framework. The metal–N/Cl coordination was clearly visible — the “secret sauce” behind their remarkable performance.
What Makes These Materials So Special
The resulting single-atom catalysts exhibited exceptional activity across multiple applications.
1. Water Purification
A version containing iron (Fe₁CNCl) showed outstanding ability to degrade micropollutants in water. For instance, it completely removed the antibiotic sulfamethoxazole (SMX) within just three minutes — a rate about 35 times faster than comparable carbon materials without the metal sites.
2. Clean Energy Applications
The materials also proved highly efficient in electrocatalytic reactions, such as:
- CO₂ Reduction:
The nickel-based catalyst (Ni₁CNCl) achieved an impressive 97.4% Faradaic efficiency for converting CO₂ into CO at a current density of 200 mA/cm². - Oxygen Reduction Reactions (ORR):
These reactions are crucial in fuel cells and metal–air batteries. The SACs displayed significant improvements in activity and stability compared to conventional catalysts.
3. Scalability and Versatility
Perhaps the most exciting part is that this process works on mixed plastics, not just single-polymer waste. And with yields ranging from 18% to 50%, it shows strong potential for practical adoption.
Why Single-Atom Catalysts Matter
Single-atom catalysts are one of the hottest frontiers in chemistry and materials science. They bridge the gap between homogeneous (molecular-level) and heterogeneous (solid-surface) catalysis, combining the best of both worlds: high activity, selectivity, and durability.
Because each atom is an active site, there’s zero waste of metal, which is critical when using precious or rare metals. And when supported on carbon frameworks, these catalysts can handle harsh conditions while maintaining their atomic dispersion.
The main challenge in SAC research has always been how to make them at scale without losing atomic precision. That’s why this study is a major leap forward — it offers a simple, universal, and recyclable method to produce SACs from cheap, abundant waste materials.
Environmental and Industrial Significance
This approach could fundamentally change how we deal with plastic waste. Instead of seeing plastic as an environmental burden, it becomes a valuable raw material for the next generation of clean technologies.
Here’s why this breakthrough matters:
- Tackles Plastic Pollution:
It offers a real way to upcycle plastics rather than just recycle or burn them. - Advances the Circular Economy:
By giving waste plastics a new, high-value life, it closes the loop — turning environmental problems into economic opportunities. - Boosts Clean Energy Systems:
The catalysts can improve the efficiency of fuel cells, batteries, and CO₂ reduction systems, directly supporting global decarbonization efforts. - Affordable and Sustainable:
The materials are low-cost, the salt template can be recovered, and the process is adaptable to various feedstocks.
Challenges and Future Directions
While this is an exciting step forward, there are still hurdles before the method can be widely adopted.
- Real-World Waste Streams:
Actual plastic waste contains additives, colorants, and contaminants that could interfere with the process. Further studies are needed to confirm performance using uncleaned, mixed municipal waste. - Energy and Cost Balance:
High-temperature pyrolysis (~900°C) requires significant energy input. Researchers must ensure that the net environmental impact is positive when scaling up. - Durability and Reusability:
Long-term stability, regeneration, and recycling of the catalysts must be tested, especially in industrial water treatment and energy systems. - Industrial Scale-Up:
Producing SACs on the gram scale is impressive, but commercial adoption will require kilogram or ton-level processes, along with efficient collection and preprocessing of waste plastics. - Economic Viability:
The choice of metals will matter. Transition metals like Fe and Ni are cheap and abundant, but precious metals might not be practical for large-scale use.
Despite these challenges, the study provides a clear proof of concept that high-value materials can be made directly from plastic waste — a vision that aligns with global sustainability goals.
A Quick Dive into Carbon Nanomaterials
To appreciate this breakthrough, it helps to understand what carbon nanomaterials are.
Carbon can form a wide variety of structures — graphene, nanotubes, fullerenes, and porous carbons — all with unique electrical, mechanical, and chemical properties. These structures are prized for:
- High surface area (great for catalysis and adsorption)
- Excellent electrical conductivity
- Chemical tunability
- Mechanical strength
When doped with heteroatoms like nitrogen or chlorine, or decorated with single metal atoms, carbon nanomaterials become versatile platforms for reactions ranging from pollutant breakdown to energy conversion.
By turning plastics into these advanced carbon frameworks, the researchers essentially converted waste into wealth — creating materials that could outperform traditional catalysts and electrodes.
The Bigger Picture: Synchrotron Science and Collaboration
This work also highlights the power of synchrotron science. At ANSTO’s Australian Synchrotron, scientists used X-ray Absorption Spectroscopy (XAS) to uncover how the metal atoms were bonded and why the catalysts performed so well. Such detailed atomic insights are crucial for improving performance and designing new materials.
The collaboration between the University of Adelaide (led by Professor Shaobin Wang and Associate Professor Xiaoguang Duan) and ANSTO underscores how interdisciplinary partnerships — combining chemistry, materials science, and advanced instrumentation — can produce real solutions to global challenges.
Closing Thoughts
This discovery is more than just a clever way to recycle plastic. It’s a blueprint for rethinking waste. If plastic pollution is one of the defining environmental crises of our time, then this research shows a path forward — where yesterday’s garbage fuels tomorrow’s clean technology.
By transforming waste plastics into single-atom carbon nanomaterials, scientists are proving that even our biggest environmental problems can become the foundation of sustainable innovation.
Research Reference:
Salt-templated transformation of waste plastics into single-atom catalysts for environmental and energy applications – Nature Communications (2025)