Turning Wood Waste into Light: Yale Scientists Create Eco-Friendly Photoluminescent Materials
A team of researchers from Yale University has developed an entirely new way to make photoluminescent materials—the kind that glow when exposed to light—using waste from the paper industry and a common amino acid found in living organisms. Their approach replaces toxic, non-renewable chemicals with renewable, safe ingredients.
The study, led by Ho-Yin (Leo) Tse, with Professors Julie Zimmerman and Paul Anastas, and co-authored by Hanno Erythropel, was published in Chem (2025) under the title Renewably sourced amino-acid- and lignin-based solid-state emitters.
This work could mark a turning point for sustainable photoluminescent technology, showing that natural materials can emit light efficiently without relying on harmful or rare elements.
The Problem with Traditional Photoluminescent Materials
Modern devices—from LED displays and smartphone screens to solar cells, optical sensors, and biomedical imaging tools—depend on materials that can absorb and re-emit light. These are known as photoluminescent materials, and they play a crucial role in how we generate, detect, and display light.
However, most of the materials currently in use are environmentally hazardous. They rely heavily on heavy metals and halogenated compounds such as:
- Cadmium-based quantum dots (used in many LED displays)
- Platinum or iridium complexes (used in organic light-emitting diodes, or OLEDs)
- Lanthanide systems (used in sensors and imaging)
These materials are problematic for several reasons:
- Toxicity Risks: During manufacturing, workers may be exposed to corrosive chemicals, volatile solvents, or toxic metal salts.
- Environmental Impact: Mining and refining these metals are energy-intensive processes that harm ecosystems and contribute to pollution.
- End-of-Life Waste: When electronics containing these materials are discarded, they can release hazardous substances into the environment if not properly recycled.
Even though most consumers face low exposure under normal conditions, the full life cycle of these materials—from extraction to disposal—poses major sustainability challenges.
The Yale Team’s Solution: Lignin and Histidine
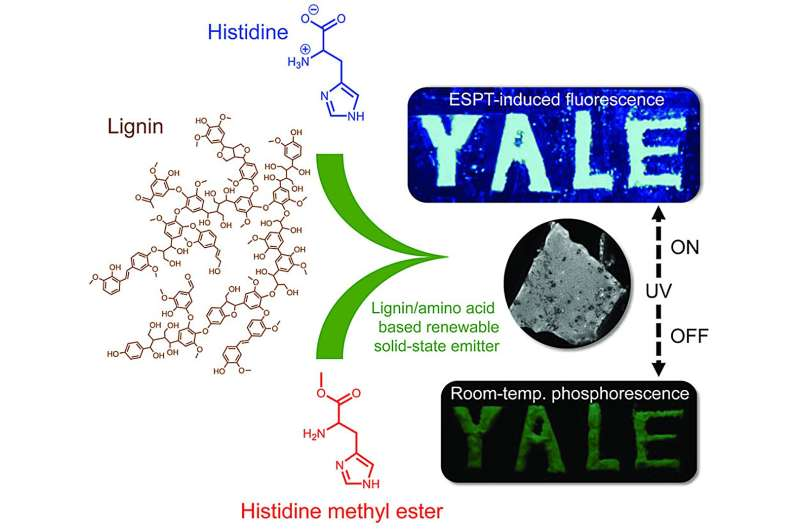
Instead of turning to metals or synthetic chemicals, the Yale researchers looked to nature for inspiration. They developed solid-state photoluminescent materials made entirely from lignin and histidine.
Lignin is a complex organic polymer found in the cell walls of plants. It gives wood its stiffness and helps plants resist decay. When making paper, lignin is separated from cellulose and often ends up as a waste product that’s simply burned for energy. Despite this, lignin is rich in aromatic rings—chemical structures that can absorb and emit light.
In the chemical industry, aromatic compounds are usually derived from petroleum, but lignin offers a renewable and carbon-neutral alternative. Using lignin for high-value materials like photoluminescent emitters could transform it from a waste byproduct into a valuable resource.
Histidine, on the other hand, is one of the twenty standard amino acids that make up proteins in living organisms. It contains an imidazole group, a ring structure that plays an important role in many biological reactions. When combined with lignin, histidine contributes to the emission process by interacting with the lignin’s aromatic groups, leading to visible luminescence.
The resulting material forms a solid-state emitter, meaning it glows without needing to be dissolved in a liquid or embedded in a complex matrix. Importantly, the synthesis requires no heavy metals, no halogens, and no high-temperature or energy-intensive steps.
How It Works
When the lignin-histidine composite is exposed to ultraviolet (UV) light, it absorbs energy and re-emits it as visible light. The researchers found that the afterglow—the time it continues to glow after the light source is removed—lasts about 300 milliseconds. That’s roughly a third of a second, enough to be visible to the human eye.
While this may sound short, it’s a strong result for a material made solely from renewable, non-toxic sources. More importantly, the researchers see room for significant improvement. By experimenting with different types of lignin (since lignin’s composition varies among plant species) and adjusting the amino-acid structure, they believe both fluorescence (instantaneous glow) and phosphorescence (afterglow) can be enhanced.
In some variations, replacing the hydrogen atom in histidine with a methyl group produced longer-lasting phosphorescence with a greenish afterglow, visible after the UV light was switched off.
The study also showed that solid-state packing—how the molecules are arranged—matters greatly. The lignin and histidine need to be arranged closely enough to transfer energy efficiently but not so tightly that they quench each other’s glow. The balance between these factors determines how bright and long-lasting the luminescence is.
Why This Matters
This research matters for several big reasons:
- It reduces dependence on toxic metals and rare elements, paving the way for greener electronic materials.
- It adds value to waste, converting industrial byproducts into advanced materials.
- It demonstrates the potential of bio-based chemistry, showing that natural substances can perform complex optical functions usually reserved for synthetic materials.
The approach fits squarely within the field of green chemistry, which focuses on designing materials and processes that minimize environmental harm. In fact, one of the senior authors, Paul Anastas, is often called the “father of green chemistry,” and this work continues that tradition by merging sustainability with high-tech innovation.
The Bigger Picture: What Is Photoluminescence?
To understand the importance of this research, it helps to know what photoluminescence is.
Photoluminescence occurs when a material absorbs photons (light energy) and then emits new photons at a different wavelength. There are two main types:
- Fluorescence: The material emits light almost immediately after absorbing it—within nanoseconds.
- Phosphorescence: The emission happens more slowly, lasting milliseconds or even seconds after the light source is gone.
Common examples include glow-in-the-dark stickers, fluorescent dyes, and LED screens. In each case, materials that can efficiently absorb and re-emit light are key to the effect.
In high-tech devices, these materials are engineered for specific brightness, color, and lifetime. However, until now, most relied on rare-earth metals or complex organic compounds made from fossil fuels.
The Yale team’s discovery suggests that biomass-derived materials could one day compete in this space, offering a more sustainable option without sacrificing performance.
Future Directions
Although the prototype materials are still in the early stages, the research opens up several exciting possibilities:
- Optimizing Composition: Different types of lignin and amino acids could yield better brightness or longer afterglow.
- Device Integration: With further refinement, these materials could be used in displays, sensors, or security markings.
- Circular Manufacturing: Using renewable feedstocks like lignin could make photoluminescent materials part of a closed-loop economy.
There are challenges ahead—such as improving light intensity, stability, and color tuning—but the proof of concept is strong. This could inspire a new class of renewable photonic materials that combine environmental responsibility with practical performance.
The Takeaway
The idea of turning tree waste into light captures both the simplicity and the brilliance of this research. By combining waste lignin with a naturally occurring amino acid, Yale scientists have created a glowing material that’s safe, renewable, and full of potential.
It’s a reminder that sometimes the answers to our high-tech problems are waiting quietly in the natural world—if we know where to look.
Research Reference:
Ho-Yin Tse et al., “Renewably sourced amino-acid- and lignin-based solid-state emitters,” Chem (2025). DOI: 10.1016/j.chempr.2025.102781