Unlocking “Sticky” Chemistry: How Understanding CO Adsorption Could Lead to Cleaner, More Efficient Fuels
Chemists have taken a big step toward making cleaner, more efficient fuels by figuring out how to measure how tightly carbon monoxide (CO) sticks to the surface of catalysts. This “stickiness” — known as CO adsorption energy — is one of the most critical factors that determines what kind of chemical products form when carbon dioxide (CO₂) is converted into useful fuels like methanol or ethanol.
A team of researchers from The Ohio State University, led by Zhihao Cui (a postdoctoral researcher) and Anne Co (professor of chemistry and biochemistry), has developed a new experimental framework that finally allows scientists to measure CO adsorption under real reaction conditions. Until now, this has been one of the biggest missing pieces in understanding how CO₂ is turned into energy-rich fuels.
Their work, published in Nature Catalysis (2025), offers an experimental bridge between theory and practice — a way to connect computer predictions with what actually happens during electrochemical reactions.
The Core Idea: Measuring the “Stickiness” of Carbon Monoxide
When CO₂ is converted into fuel through a process called electrocatalytic reduction, it first forms carbon monoxide (CO) as an intermediate. How tightly this CO sticks to the catalyst surface can determine whether the reaction stops there or continues to form more complex molecules like ethanol or ethylene.
This sticking strength — or adsorption free energy — has always been difficult to measure experimentally. In theory, researchers could calculate it using quantum models, but real electrochemical environments are far more complex. Electrodes interact with ions, water, and applied voltages, all of which influence how molecules behave.
The Ohio State team used a widely accessible electroanalytical technique called rotating ring-disk electrode (RRDE) voltammetry combined with a kinetic analysis model. This combination allowed them to measure how CO binds to a catalyst surface under true reaction conditions and to relate that behavior to factors like voltage, catalyst type, and surface structure.
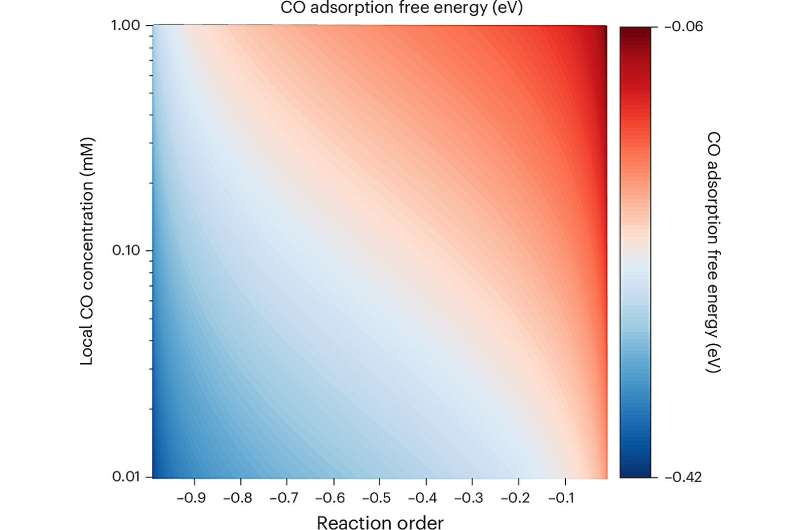
Essentially, their approach tracks how much CO is produced, how fast it reacts, and how local concentrations of CO change — all in real time. From those observations, the team could extract values for the CO adsorption free energy (ΔG₍ads₎ CO).
What They Found: Gold vs. Copper
The researchers tested their new framework on well-known catalysts such as gold (Au) and copper (Cu). These metals are standard choices in CO₂ reduction research because both can convert CO₂ into CO, but only copper is capable of going a step further — forming multi-carbon (C₂⁺) products like ethylene and ethanol.
Surprisingly, the study found that gold and copper bind CO with almost the same strength. This means that the difference in CO adsorption energy cannot alone explain why copper is much better at producing multi-carbon fuels.
This finding challenges a long-standing assumption in catalyst design — that having the “right” CO binding energy is the key to forming complex fuel molecules. Instead, the researchers discovered that CO adsorption is influenced by a mix of factors, including:
- The type of metal and its surface structure (e.g., rough vs. smooth, crystal orientation)
- The ion identity and concentration in the surrounding electrolyte
- The applied voltage and resulting electrochemical potential
All these variables affect the local CO concentration at the catalyst surface, which in turn changes the reaction order (a measure of how the rate depends on CO concentration).
Why This Matters
Understanding how CO behaves on catalyst surfaces is crucial for building better systems that turn CO₂ into fuels. CO₂ is a very stable molecule, meaning it takes a lot of energy to break its bonds and rearrange its atoms into something useful.
By precisely measuring CO adsorption energies, researchers can design catalysts that lower these energy requirements. Better catalysts mean more efficient fuel production and potentially greener alternatives to fossil fuels.
This new framework also helps validate theoretical predictions. Before now, scientists relied heavily on computer models (like density functional theory, or DFT) to guess how CO would bind to different materials. But without real experimental data under operating conditions, those guesses had limits. The new method gives scientists a tool to check and refine their models.
Perhaps most exciting is that this experimental setup is relatively simple and inexpensive. It doesn’t require advanced spectroscopy or specialized facilities, which means labs around the world can easily adapt it to test different catalysts.
The Bigger Picture: Why CO₂ Conversion Matters
The broader goal behind all of this is carbon recycling — converting carbon dioxide, a greenhouse gas, into useful chemicals and fuels. If done efficiently, it could provide a closed carbon loop, reducing emissions while generating valuable products.
The process typically works by applying an electric voltage to a catalyst submerged in an electrolyte, driving CO₂ to react and form new molecules. The challenge has always been to push this reaction beyond just CO and form energy-dense multi-carbon molecules.
Copper remains the only common metal that can do this at meaningful rates, but even it is far from perfect. Researchers are still trying to understand why copper performs so well and how to replicate its magic in other, cheaper materials.
This new study moves that effort forward by providing a new lens through which to study CO₂ conversion. It reveals that factors like local CO concentration, electrolyte composition, and applied potential may matter just as much — or even more — than the simple “binding strength” idea that’s dominated the field for years.
How This Framework Works in Practice
Here’s how the researchers carried out their analysis:
- They used RRDE voltammetry, a technique where a disk electrode generates CO from CO₂, and a surrounding ring electrode detects how much CO escapes into the solution.
- By adjusting the rotation speed and voltage, they could control and measure the local CO concentration near the electrode.
- They then observed how the reaction rate changed with CO concentration, revealing the reaction order of CO.
- Using a kinetic model, they related the reaction order and local CO concentration to determine the CO adsorption free energy (ΔG₍ads₎ CO).
This approach allowed them to measure CO adsorption in real electrochemical environments — something that had previously been considered almost impossible.
What Comes Next
The Ohio State team notes that their model still has limitations. It makes simplifying assumptions about how CO moves near the surface and how reaction steps occur. The RRDE setup also differs from industrial catalysts, which often use porous electrodes with more complex surfaces.
Still, this framework offers a strong foundation. The next steps include:
- Refining the model to capture even more detailed dynamics
- Expanding experiments to other catalysts beyond gold and copper
- Combining this kinetic approach with in-situ spectroscopic techniques to directly observe how CO interacts with catalyst surfaces in real time
The ultimate goal is to identify the true descriptors — the measurable properties that govern whether a catalyst can efficiently make multi-carbon fuels from CO₂.
The Science Behind It: A Quick Chemistry Refresher
To understand why all of this matters, it helps to know a bit about CO₂ electroreduction.
When you apply a voltage to CO₂ in the presence of a catalyst, electrons are transferred to CO₂ molecules, breaking their strong double bonds and forming new compounds. The first stable product is usually CO, but with the right conditions, further reactions can form hydrocarbons or alcohols.
Each reaction step involves adsorption (a molecule sticking to a surface), electron transfer, and desorption (the product leaving the surface). How tightly each intermediate sticks determines which pathway dominates.
For example:
- If CO binds too weakly, it leaves the surface before forming more complex molecules.
- If it binds too strongly, it blocks other reactants from getting to the surface.
That’s why understanding CO adsorption energy is so important — it’s the balancing act that dictates efficiency and selectivity.
Conclusion
This research marks a significant leap forward in the quest to convert CO₂ into sustainable fuels. By finally providing a practical way to measure CO adsorption under true working conditions, the Ohio State team has opened the door to smarter catalyst design and a deeper understanding of electrochemical carbon conversion.
Their discovery not only redefines how scientists think about CO₂ reduction but also highlights how even “simple” measurements, when designed cleverly, can unlock entirely new directions in clean energy research.
Research Reference: Determining CO adsorption free energies on CO₂ electroreduction active sites through kinetic analysis – Nature Catalysis (2025)





