The Electrifying Science Behind Martian Dust Is Revealing a Surprisingly Active Red Planet
Mars has long been described as a cold, dry, and mostly inactive world, but new research is steadily reshaping that image. Far from being a silent desert, the Red Planet hosts an energetic and electrically charged environment, driven largely by its ever-present dust. Recent scientific work is now showing that Martian dust storms and dust devils are not just weather events—they are powerful chemical engines capable of reshaping Mars’s surface and atmosphere.
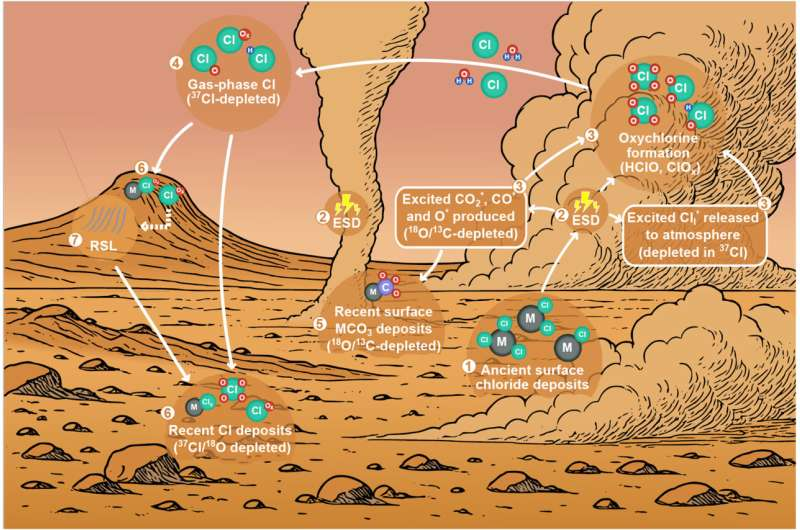
At the center of this research is planetary scientist Alian Wang, whose latest study dives deep into how dust-driven electrical activity affects Mars at a fundamental chemical level. Published in Earth and Planetary Science Letters, the study examines the isotopic geochemical consequences of electrostatic discharges generated by Martian dust activity, offering fresh insight into how modern Mars works.
Why Martian Dust Is Electrically Active
Mars is famous for its dust storms, some of which can engulf the entire planet. When dust grains collide and rub against each other in Mars’s thin, low-pressure atmosphere, they become electrically charged through a process known as frictional or triboelectric electrification. As these charges build up, they can create electrical potentials strong enough to trigger electrostatic discharges (ESDs).
Unlike Earth, where dense air limits how easily electrical breakdown occurs, Mars’s atmosphere allows these discharges to happen more readily. Instead of dramatic lightning bolts, Martian ESDs likely appear as faint glows or localized electrical breakdowns, similar in spirit to auroral activity. These subtle events may look harmless, but chemically, they are extremely powerful.
How Electrical Discharges Drive Martian Chemistry
Wang’s research focuses on what happens when these electrical discharges interact with dust, gas, and surface materials. Her team built two advanced planetary simulation chambers—PEACh (Planetary Environment and Analysis Chamber) and SCHILGAR (Simulation Chamber with InLine Gas AnalyzeR)—designed to replicate Martian atmospheric pressure, composition, and dust conditions.
Inside these chambers, simulated dust-driven electrical discharges produced a surprisingly diverse set of chemical compounds, including:
- Volatile chlorine species
- Activated metal oxides
- Airborne carbonate minerals
- Chlorates and perchlorates
These substances are not theoretical. Many of them have already been detected by Mars orbiters, rovers, and landers, making the laboratory results directly relevant to real Martian observations.
Rewriting the Chlorine Story on Mars
One of the most important outcomes of this research is its impact on our understanding of Mars’s chlorine cycle. The Martian surface is scattered with chloride deposits, remnants of ancient salty waters. For years, scientists struggled to explain how Mars also ended up with large quantities of perchlorates, highly oxidized chlorine compounds found by multiple missions.
Wang’s earlier studies showed that dust-induced electrical discharges could drive chlorine chemistry even during the planet’s hot and dry Amazonian period, long after surface water disappeared. By carefully trapping and measuring reaction products in simulation experiments, her team demonstrated that dust activity alone could generate chlorine-bearing compounds in quantities consistent with spacecraft observations.
The new study goes further by examining the isotopic fingerprints of these products.
Isotopes as Chemical Evidence
Isotopes are atoms of the same element with different weights, and they act like chemical fingerprints. Because isotopes are minor components, their ratios are only altered by dominant processes in a system. In this study, Wang’s team measured isotopes of chlorine, oxygen, and carbon in the ESD-generated products.
The results were striking. The researchers found strong and consistent depletion of heavy isotopes across all three elements. This pattern points directly to dust-driven electrochemistry as a major force shaping Mars’s surface–atmosphere system.
On Mars, NASA’s Curiosity rover has measured an extremely negative δ37Cl value of –51‰, indicating unusually light chlorine. The study shows that ongoing dust electrochemistry during the Amazonian period could progressively deplete heavy chlorine isotopes, pushing values in exactly the direction observed on the planet today.
A New Model for Mars’s Surface–Atmosphere Cycle
Using these isotopic findings, the researchers developed a conceptual model of Mars’s contemporary global chlorine cycle. In this model:
- Dust storms and dust devils generate electrical discharges.
- These discharges produce reactive chlorine, oxygen, and carbon compounds.
- The products enter the atmosphere and are transported globally.
- They eventually settle back onto the surface, becoming part of secondary minerals.
- Some materials percolate into the subsurface, forming future generations of surface deposits.
This process links atmospheric chemistry, surface minerals, and subsurface evolution into a single, electrically driven system.
Real Mars Observations Support the Lab Results
The timing of Wang’s study aligns closely with new findings from NASA’s Perseverance rover, which recently recorded 55 electrical discharge events associated with dust devils and dust storm fronts on Mars. These observations, published in Nature, provide direct evidence that electrical activity is not just theoretical—it is actively happening on Mars today.
Earlier work by Wang was cited in these rover studies as the likely explanation for the chemical consequences of such electrical events, reinforcing her role as a leading expert on Mars’s electrified environment.
Why This Matters for Mars and Beyond
This research fundamentally changes how scientists view modern Mars. Rather than a chemically frozen planet, Mars appears to be chemically dynamic, with dust-driven electrochemistry continuously reshaping its surface and atmosphere.
It also helps explain the presence of perchlorates, which are important for two reasons. On one hand, they can destroy organic molecules, complicating the search for past life. On the other, they can serve as potential energy sources for microbes, making them relevant to astrobiology.
Beyond Mars, the implications stretch across the solar system. Similar electrochemical processes may occur on Venus, where lightning is suspected, on the Moon, where dust is electrically charged, and on outer planetary moons exposed to energetic particles. Wherever dust, low pressure, and electrical energy coexist, similar chemistry may follow.
What We Now Know About Martian Dust
Thanks to this growing body of research, Martian dust is no longer just a nuisance for solar panels and rover wheels. It is a powerful agent of planetary change, capable of driving chemical reactions, altering isotopic signatures, and linking the surface and atmosphere in ways previously overlooked.
Mars, it turns out, is not just red. It is electrically alive, chemically active, and still full of surprises.
Research Paper Reference:
https://doi.org/10.1016/j.epsl.2025.119784





