AI and High-Throughput Testing Reveal Stability Limits in Organic Redox Flow Batteries
Artificial intelligence and high-throughput experimentation are steadily changing how scientific research is done, and a recent study from researchers at the U.S. Department of Energy’s Argonne National Laboratory is a strong example of just how powerful this combination can be. By pairing robotics, automation, and machine learning, the team uncovered a fundamental limitation in a promising energy storage technology: organic redox flow batteries.
The finding is important not because it announces a breakthrough battery ready for commercialization, but because it clearly defines a stability ceiling that researchers around the world have been struggling to overcome for years. Understanding where that limit lies could save enormous amounts of time, money, and effort—and redirect future battery research toward more realistic and creative solutions.
What Are Organic Redox Flow Batteries?
Redox flow batteries (RFBs) are rechargeable energy storage systems designed primarily for large-scale, long-duration applications, such as storing electricity for power grids. Unlike lithium-ion batteries, which store energy inside solid electrodes, RFBs store energy in liquid electrolytes kept in external tanks.
Organic redox flow batteries are a specific class of RFBs that use carbon-based (organic) molecules instead of metal ions. This makes them attractive for several reasons:
- Organic compounds are generally abundant and inexpensive
- They offer the potential for low-cost, scalable energy storage
- Non-aqueous organic systems can operate at higher voltages than water-based flow batteries
- Higher voltage means higher energy density for the same physical footprint
For years, these advantages have made organic RFBs a promising candidate for reinforcing renewable-heavy electricity grids. However, one major obstacle has refused to go away: poor long-term stability.
The Stability Problem Researchers Couldn’t Solve
At the heart of every redox flow battery are charged molecules that undergo oxidation and reduction reactions to store and release energy. In organic RFBs, these charged organic molecules tend to be highly reactive, especially at the higher voltages that make them attractive in the first place.
When these molecules react in unintended ways, they degrade. Once degraded, their charge can no longer be recovered, and the battery slowly loses its capacity. For grid-scale energy storage, which demands years or even decades of reliable operation, this instability is unacceptable.
Despite years of global research, progress on stability improvements has been limited. Many scientists suspected there might be a fundamental chemical barrier, but proving it using traditional, slow experimental methods would have taken decades.
Using AI and Automation to Ask a Big Question
The Argonne team decided to tackle the problem differently. Their central question was simple but ambitious: Can choosing the right solvent dramatically improve the stability of charged organic molecules?
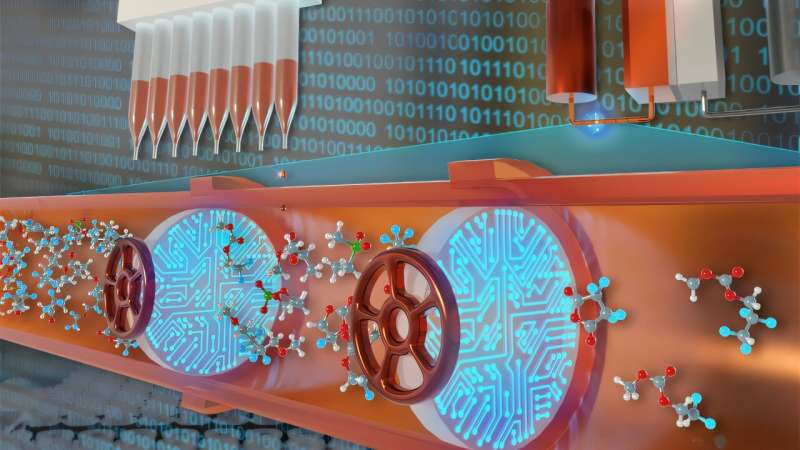
The challenge was scale. There are hundreds of possible organic solvents, and testing them one by one under controlled conditions would normally take many years. Instead, the researchers turned to one of Argonne’s Robotic Autonomous Platforms for Innovative Discovery (RAPID) laboratories.
Within this automated setup, robots prepared samples, ran experiments, and collected data, while machine learning algorithms analyzed results in real time and guided what should be tested next.
In just five months, the system carried out more than 6,000 experiments, a workload that would have taken five to eight years using conventional lab techniques.
How the Experiments Worked
The researchers focused on a charged organic molecule called methylphenothiazine (MPT), a well-studied redox-active compound. Using nuclear magnetic resonance spectroscopy, they tracked how MPT behaved when mixed with different solvents.
Early on, they observed something critical: instead of remaining stable, the charged molecules were fragmenting solvent molecules, causing themselves to become neutralized in the process. This meant the solvent was not just a passive medium—it was actively involved in degradation reactions.
To quantify this behavior, the team tested MPT with hundreds of solvents drawn from chemical databases, industrial solvent lists, and commercial catalogs. Because the materials were sensitive to oxygen and moisture, all experiments were conducted using a liquid-handling robot inside an air-free glovebox.
Samples were assembled on microplates containing 384 wells each, and two plate-reader spectrophotometers monitored color changes over time. These color changes revealed how quickly the charged molecules decayed in each solvent.
Machine learning models compared reaction rates to a baseline solvent, eliminating unpromising options and directing attention toward the most informative experiments. This approach allowed the team to characterize 540 solvents while physically testing only about one-third of them.
A Sobering Result: The Stability Barrier Is Real
The outcome was clear and unexpected in its consistency. Most solvents followed similar degradation pathways, and only three solvents showed a meaningful improvement over the baseline. Even then, the improvement was modest.
In short, changing the solvent alone does not solve the stability problem.
This confirmed what many researchers had suspected but could not prove: there is a fundamental molecular-level limit governing the stability of charged organic species in non-aqueous electrolytes. Beyond a certain point, chemical degradation becomes unavoidable.
This insight explains why years of incremental progress have learned so little. The field was pushing against a wall it could not see—until now.
Why This Finding Matters
By clearly identifying the stability limit, the study reshapes how organic redox flow battery research should move forward. Instead of endlessly searching for the “perfect” long-lived organic molecule, researchers may need to rethink their assumptions entirely.
Some promising directions include:
- Designing batteries for shorter operational lifetimes with planned material reuse
- Repurposing degraded organic materials for industrial or agricultural applications
- Applying the most stable solvents identified in this study to other battery chemistries, such as sodium-ion or lithium-metal systems
- Using autonomous discovery platforms to explore entirely new electrolyte concepts
Just as importantly, the work demonstrates how AI-driven experimentation can rapidly answer questions that would otherwise consume decades of scientific effort.
High-Throughput Science and the Future of Battery Research
This study is part of a broader shift toward autonomous discovery, where robotics and AI handle repetitive experimentation while humans focus on interpretation and strategy. At Argonne, this approach is being applied not only to batteries, but also to critical materials, microelectronics, and quantum systems.
For energy storage research in particular, the implications are significant. As renewable energy deployment accelerates, the need for reliable, affordable, long-duration storage becomes more urgent. Knowing which paths are dead ends is just as valuable as finding new breakthroughs.
By revealing the invisible stability barrier in organic redox flow batteries, this research provides the global battery community with a much clearer map of the chemical landscape—and a better sense of where innovation is still possible.
Research Paper Reference:
https://doi.org/10.1021/jacs.5c10140