How LLZTO’s Unique Atomic Structure Helps the Next Generation of Solid-State Batteries Stay Cool
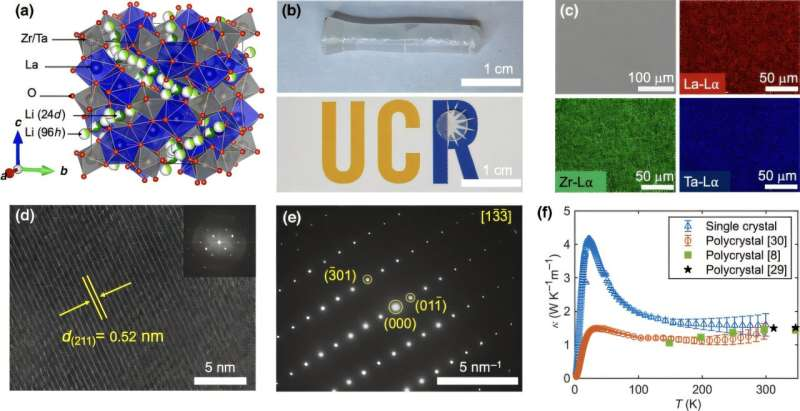
A team of engineers from the University of California, Riverside has uncovered why a promising solid-state battery material, known as LLZTO (lithium lanthanum zirconium tantalum oxide), stays remarkably cool even when ions are zipping through it at high speed. This new understanding could help scientists design safer, more powerful, and thermally stable solid-state batteries—one of the most anticipated technologies in energy storage.
LLZTO is a garnet-type ceramic solid electrolyte, meaning it acts as the layer between the cathode and anode inside a battery, but unlike the flammable liquid electrolytes found in today’s lithium-ion batteries, it’s a solid material with far lower fire risk. Solid electrolytes like LLZTO are crucial for the future of lithium metal batteries, which promise much higher energy density than today’s lithium-ion cells.
However, even as LLZTO is known for excellent ionic conductivity and safety, researchers have long been puzzled by its unusually low thermal conductivity—a characteristic that determines how well a material can transfer heat. Understanding why LLZTO doesn’t carry heat efficiently is key to predicting how batteries behave during charging and discharging, especially under heavy load.
The UC Riverside team’s findings were published in PRX Energy, in a study titled Origin of Intrinsically Low Thermal Conductivity in a Garnet-Type Solid Electrolyte: Linking Lattice and Ionic Dynamics with Thermal Transport. The work finally explains what’s happening at the atomic level.
LLZTO’s Naturally Low Thermal Conductivity
Thermal conductivity describes how well a material can move heat from one place to another. Metals like copper have high thermal conductivity, which is why they’re used in heat sinks and electronics cooling. LLZTO, however, has a thermal conductivity of only 1.59 watts per meter-kelvin—about 250 times lower than copper.
To determine whether this low thermal conductivity comes from defects or is built into the material itself, the researchers grew single crystals of LLZTO using a floating-zone method. Single crystals are free of grain boundaries and imperfections, so any measured property reflects the material’s true intrinsic behavior.
Even in this pristine form, the material still had extremely low thermal conductivity. That confirmed the low heat flow is inherent to LLZTO, not caused by defects in manufactured samples.
This finding matters because when batteries charge or discharge, they generate heat. If the electrolyte overheats or conducts heat inefficiently, temperature spikes can degrade battery materials, shorten lifespan, or trigger thermal runaway—where a cell enters a dangerous chain reaction. Because airlines and regulatory bodies strictly limit battery transport due to these risks, understanding how heat moves inside future batteries is essential.
Why LLZTO Stays Cool: What’s Happening at the Atomic Scale
To reveal the cause of LLZTO’s low thermal conductivity, the team used a mix of experimental and computational tools. This included neutron scattering experiments at Oak Ridge National Laboratory as well as advanced simulations of atomic vibrations.
In solid materials, heat is carried mostly by phonons, which are vibrations of atoms within a crystal lattice. There are two major types:
- Acoustic phonons – the main carriers of heat
- Optical phonons – higher-frequency vibrations that don’t carry heat efficiently
Two findings from the study explain LLZTO’s low heat flow:
1. Abundant Optical Phonon Modes Scatter Heat-Carrying Phonons
LLZTO’s crystal structure naturally produces a large number of optical phonon modes. These optical vibrations interact with acoustic phonons and scatter them—basically shaking them off course.
More scattering means phonons travel shorter distances before colliding, which sharply reduces thermal conductivity.
This is unusual because LLZTO is a perfectly ordered crystal, yet it behaves more like a glass when it comes to heat movement. In most crystals, phonons move freely and carry heat well, but LLZTO’s internal complexity disrupts this motion.
2. Strong Anharmonicity Further Disrupts Heat Transport
Another key factor is the material’s anharmonicity—a measure of how much atomic vibrations deviate from ideal harmonic motion.
LLZTO has large anharmonicity, driven in part by the presence of mobile lithium ions, which do not stay fixed in one spot. This irregular movement introduces additional scattering effects, preventing phonons from traveling long distances.
Because both mechanisms act together, the usual models used to describe heat flow in crystals don’t fully apply here. LLZTO behaves in a hybrid manner, somewhere between a crystal and a disordered glass.
Why This Matters for Next-Generation Batteries
Solid-state batteries promise:
- higher energy density
- better safety
- compatibility with lithium-metal anodes
- reduced fire risk
But with increased energy density comes more heat generation. An electrolyte that naturally restricts heat flow can help maintain stable temperature zones and reduce the risk of overheating near sensitive components.
At the same time, low thermal conductivity can also make it difficult to dissipate heat outward, meaning engineers must carefully design how heat is removed from whole battery packs.
Understanding LLZTO’s thermal behavior gives researchers a clearer way to:
- predict temperature distribution inside cells
- decide how thick the electrolyte layer should be
- optimize battery charging speeds
- prevent degradation caused by temperature gradients
- customize materials for superior performance
This research offers guidelines for designing future electrolytes that combine high ionic conductivity with controlled thermal transport, improving both performance and safety.
Additional Context: What Makes Garnet-Type Electrolytes Special?
The LLZO family of electrolytes, including LLZTO, stands out because of:
- high lithium-ion conductivity
- chemical stability in air compared to sulfide electrolytes
- compatibility with lithium metal
- mechanical strength that helps suppress lithium dendrites
LLZTO specifically adds tantalum (Ta) to stabilize the cubic phase, which allows for faster lithium movement.
Because of these strengths, LLZTO-based electrolytes are being explored for:
- electric vehicle batteries
- grid-scale energy storage
- portable electronics
- aerospace applications
This latest study enhances the understanding of how these materials behave thermally—an area that previously received less attention than ionic conductivity.
Additional Insight: Why Thermal Conductivity Matters in Battery Engineering
Every time a battery charges or discharges, heat is produced from:
- ionic resistance
- electronic resistance
- interfacial reactions
- mechanical stress
If heat builds up unevenly, it leads to:
- hot spots
- faster degradation
- uneven lithium plating
- potential short circuits
- thermal runaway
Understanding how heat travels through every layer of a battery—electrolyte, electrodes, interfaces—is critical for building high-power solid-state batteries. Low thermal conductivity materials like LLZTO must be matched with good heat dissipation strategies at the pack level, such as:
- thermal pads
- cooling plates
- optimized cell spacing
- external heat sinks
This research adds a crucial piece to that engineering puzzle.
Research Paper Reference
Origin of Intrinsically Low Thermal Conductivity in a Garnet-Type Solid Electrolyte: Linking Lattice and Ionic Dynamics with Thermal Transport
https://doi.org/10.1103/6wj2-kzhh