PFAS-Free Ultra-Thin Membranes With Nanoscopic Plugs Could Make Hydrogen Production Cleaner, Safer, and Cheaper
Hydrogen already plays a massive role in modern industry. It fuels a $250-billion global sector, supporting fertilizer production, steelmaking, oil refining, and a long list of chemical processes. But there’s a problem that’s becoming increasingly hard to ignore: almost all the hydrogen we rely on today is produced using carbon-heavy methods. As the world pushes toward cleaner energy, researchers are racing to make water electrolysis—the process of splitting water into hydrogen and oxygen using electricity—more efficient, affordable, and environmentally responsible.
A team led by Dan Esposito, a chemical engineer at Columbia University, has taken a major step toward that goal. Working with industrial partners Nel Hydrogen and Forge Nano, the team is developing a new kind of electrolyzer membrane that could drastically reduce environmental risks while improving performance. Their key innovation: an ultra-thin, PFAS-free silicon dioxide membrane strengthened with nanoscopic “plugs” that seal microscopic cracks.
Below is a clear, thorough breakdown of what they created, why it matters, and how it could change the future of hydrogen.
A Quick Look at Why Electrolyzer Membranes Matter
Electrolyzers rely on a thin membrane to do two crucial jobs at once:
- Block hydrogen and oxygen gases from mixing.
- Let positively charged hydrogen atoms (protons) pass through.
This membrane is literally the heart of the electrolyzer. If it fails, gases mix, the system becomes inefficient, and under certain conditions, the mixture can even become explosive.
Right now, the industry standard membrane is Nafion, a type of PFAS (per- and polyfluoroalkyl substance). PFAS chemicals are often called “forever chemicals” because they persist in the environment for decades. Improper disposal or large-scale industrial use of PFAS can lead to serious environmental hazards.
That’s where the Columbia Engineering team comes in.
Replacing PFAS With Ultra-Thin Oxide Membranes
Instead of using PFAS-based polymers like Nafion, Esposito’s group turned to silicon dioxide, a safe and PFAS-free oxide material. At first glance, silicon dioxide wouldn’t seem ideal for this application. Its proton conductivity is far lower than Nafion’s. However, conductivity is only half the story.
Total resistance depends on both conductivity and thickness. So the team solved the conductivity challenge by using an advanced manufacturing technique called atomic layer deposition (ALD) to create membranes less than one micron thick—hundreds of times thinner than Nafion, which is typically around 180 microns thick.
To put that into a visual context:
- A human hair is ~70–100 microns thick.
- Nafion is roughly two to three times thicker than a human hair.
- The new oxide membrane is 1/100th the thickness of a human hair.
Because the membrane is so thin, even though silicon dioxide is less conductive, the overall electrical resistance ends up being similar to—or sometimes even approaching—the performance of the best commercial membranes.
This is a big deal because the membrane is one of the most expensive and environmentally problematic parts of an electrolyzer. Switching to a thin oxide membrane eliminates upwards of 99% of the PFAS contained in a conventional system.
The Challenge of Defects in Ultra-Thin Films
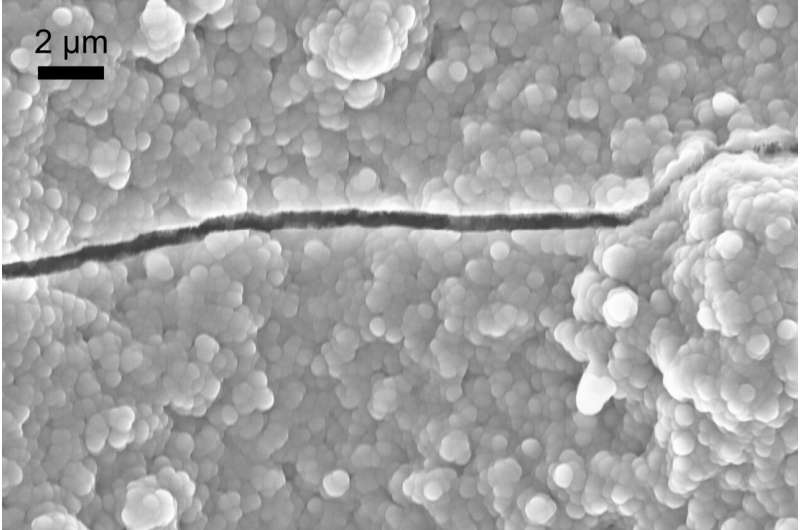
While these ultra-thin membranes have impressive potential, they come with their own challenge: defects.
Even tiny imperfections—microscopic pinholes or nano-scale cracks—can let hydrogen leak into the oxygen stream. Just a few defects per square centimeter can make the membrane unsafe. Hydrogen crossover increases explosion risk and seriously reduces efficiency.
This left the team with a key question:
How do you keep a membrane extremely thin without sacrificing safety?
The Breakthrough: Nanoscopic Plugs That Seal Cracks
The solution turned out to be wonderfully clever.
Instead of trying to eliminate defects during manufacturing (which is nearly impossible at that scale), the team developed an electrochemical defect-plugging strategy.
By applying pulsed voltage to the membrane, they triggered local chemical reactions only inside the cracks and pinholes, causing tiny amounts of material to deposit inside the defects—effectively “plugging” them.
This matters for two major reasons:
- The method preserves the ultra-thin structure.
If they used continuous current instead of pulses, material would deposit everywhere, creating a thicker, high-resistance membrane. Pulsing prevents that. - The technique is self-targeting.
The plugs form only where defects exist. No complex imaging or mapping needed.
This defect-plugging technology dramatically reduces hydrogen crossover—by up to 100× less than Nafion in early laboratory tests—even though the membrane is hundreds of times thinner.
That’s a remarkable level of safety improvement.
Early Tests and Industry Involvement
Laboratory experiments have already demonstrated that these plugged membranes:
- Maintain low electrical resistance
- Exhibit extremely low hydrogen crossover levels
- Remain intact under normal electrolyzer operation
- Can be manufactured with high precision using ALD
Because the technology shows real commercial promise, the team is now working with Nel Hydrogen and Forge Nano to scale the system from centimeter-scale prototypes to larger membranes needed for industrial electrolyzers.
Scaling is one of the biggest hurdles in clean energy innovation, so having industrial partners at this stage is a good sign.
Why This Matters for the Future of Green Hydrogen
Right now, less than 0.1% of global hydrogen is produced by water electrolysis. Most hydrogen comes from fossil-fuel-based processes like steam methane reforming, which produces large amounts of CO₂.
If the world wants to scale green hydrogen in a meaningful way, we need electrolyzers that are:
- More efficient
- Less expensive
- More environmentally friendly
- Safer at large scales
This new membrane technology checks all those boxes. Removing PFAS alone is a major step. Combining that with higher efficiency and reduced gas crossover gives the technology a strong chance of making a real impact.
Esposito also pointed out that the defect-plugging strategy isn’t limited to hydrogen electrolyzers. It could be applied to:
- Fuel cells
- Flow batteries
- Water purification systems
- Semiconductor barriers
Any field requiring ultra-thin, leak-tight membranes could potentially benefit.
Understanding the Broader Context of PFAS-Free Energy Technologies
Since this news is part of a larger push toward cleaner chemistry and energy, here’s some additional context.
What Are PFAS?
PFAS are synthetic chemicals used in everything from nonstick cookware to firefighting foam. Their biggest issue is environmental persistence. They don’t break down naturally and can accumulate in water, soil, and the human body.
Replacing PFAS in industrial components—especially ones used at large scale—is a growing priority.
Why Ultra-Thin Membranes Are Exciting
Ultra-thin membranes have huge potential in energy storage and chemical processing because they allow:
- Faster proton transport
- Lower electrical resistance
- Higher efficiency at the same voltage
- Smaller, compact device design
The problem has always been durability and defects. This new defect-plugging approach helps solve that.
Why Hydrogen Crossover Is Dangerous
Hydrogen and oxygen must remain strictly separated. Even small crossover rates can lead to:
- Reduced efficiency (lost hydrogen fuel)
- Higher operational costs
- Potentially explosive mixtures
- Faster degradation of the electrolyzer stack
A membrane 100× thinner than Nafion that still prevents hydrogen crossover is genuinely impressive.
Final Thoughts
This research is a promising move toward cleaner hydrogen production. By using PFAS-free, ultra-thin oxide membranes reinforced with nanoscopic plugs, the Columbia University team has shown that it’s possible to combine environmental responsibility, safety, and efficiency in the same device component.
There’s still a long journey ahead—from prototype to industrial scale—but the direction is encouraging. With hydrogen set to play a major role in the global energy transition, innovations like this one could make green hydrogen significantly more viable.
Research Paper:
Nanoscopic plugs block hydrogen crossover in submicron thick proton-conducting SiO₂ membranes for water electrolysis
https://pubs.acs.org/doi/10.1021/acsnano.5c09555