Reclaimed Wastewater Shows Strong Potential for Affordable and Clean Hydrogen Production

Hydrogen has been gaining global attention as a promising clean energy source, but one of its biggest hurdles is the huge amount of clean, purified water needed to produce it through electrolysis. A new study from Princeton University now suggests a practical and cost-saving alternative: using reclaimed wastewater instead of ultrapure water. The research shows that with a simple tweak, treated wastewater can reliably power hydrogen-producing electrolyzers while significantly cutting operating costs and easing pressure on freshwater resources.
This finding could reshape how industries approach green hydrogen production, especially in regions where water scarcity and industrial energy demands collide. Below is a clear and comprehensive look at exactly what the Princeton team discovered, how they solved the long-standing technical issues, and why it matters for the future of low-carbon energy.
Hydrogen Production’s Water Problem
Producing hydrogen through electrolysis—specifically green hydrogen—requires electricity to split water into hydrogen and oxygen. The process works well only when the water is extremely pure. Typical hydrogen plants start with tap water or groundwater and run it through energy-intensive purification steps like reverse osmosis and deionization.
This purification isn’t just expensive. It also requires large volumes of clean water, often competing with agricultural, industrial, or municipal water needs. The challenge becomes even bigger in water-stressed regions or regions where hydrogen plants might scale rapidly.
Most hydrogen produced today is blue hydrogen, made from natural gas with partial carbon-capture systems. But as industries push toward cleaner options, green hydrogen will need solutions that address both cost and ecological footprint.
Why Wastewater Is an Appealing Option
Every community with a wastewater treatment plant generates treated effluent—water clean enough for aquifer discharge, irrigation, or industrial cooling, but not potable. This water is widely available and often underused.
The Princeton researchers saw this as a huge opportunity. If reclaimed wastewater could feed electrolyzers without clogging or damaging them, hydrogen production could become more distributed, flexible, and affordable.
However, earlier attempts at using wastewater didn’t succeed for long. Electrolyzers tended to degrade quickly, losing efficiency and eventually failing.
The Princeton team wanted to figure out precisely why—and how to fix it.
What Exactly the Researchers Did
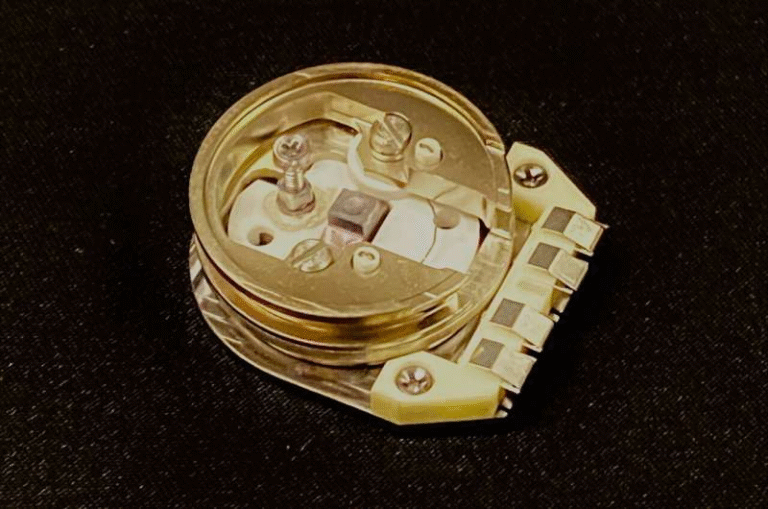
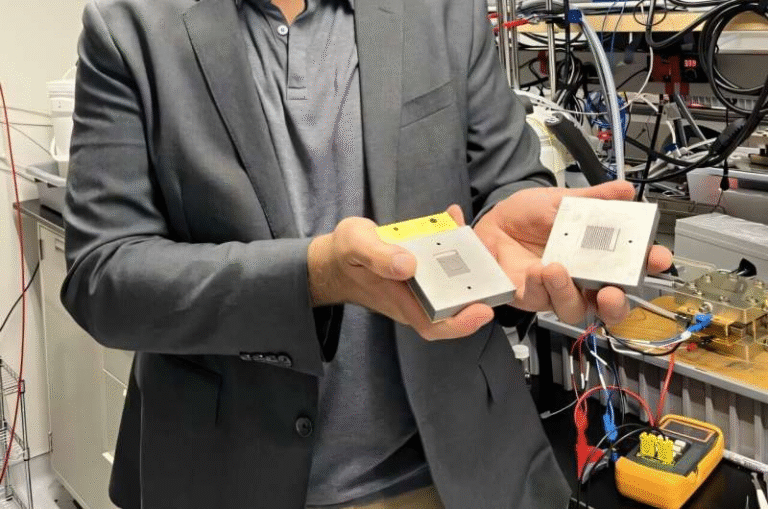
Ph.D. student Lin Du and colleagues conducted a series of controlled experiments using a proton exchange membrane (PEM) electrolyzer, the same technology used in commercial hydrogen systems. They tested three kinds of input water:
- Ultrapure water
- Treated wastewater (reclaimed water)
- Treated wastewater that had been acidified
The goal was to compare performance, longevity, and internal chemical behavior across the systems.
The Core Problem: Mineral Ions Clogging the Electrolyzer
The team discovered that reclaimed wastewater contains moderate amounts of common mineral ions—primarily calcium and magnesium. These are the same minerals that create scale on home faucets and kettles.
Inside an electrolyzer, these ions accumulate on the membrane, transforming a porous proton-transport layer into a nearly solid, blocked barrier. As a result:
- The system’s electrical resistance shoots up.
- Hydrogen production drops sharply.
- The electrolyzer fails in fewer than 8 hours of operation.
Meanwhile, the ultrapure-water system ran smoothly without issues.
Through electrochemical analysis and microscopic imaging, the team confirmed the exact mechanism of how the ions adhered to the membrane and restricted ion flow.
The Simple Fix: Acidify the Wastewater
To overcome the mineral problem, the researchers added a small amount of sulfuric acid to the reclaimed water. This created an acidic buffer with a high concentration of protons (H⁺).
Because these protons are more mobile and competitive than calcium and magnesium ions, they dominated the ion transport inside the membrane. This prevented the minerals from sticking and essentially kept the system running smoothly.
With acidified reclaimed water, the electrolyzer operated for more than 300 hours at a current density of around 150 mA per square centimeter—a dramatic improvement over all previous wastewater attempts.
Importantly:
- The acid remains inside the system, circulating continuously rather than being discharged.
- Calcium and magnesium stay dissolved in the liquid instead of precipitating or sticking to equipment.
- No major modifications were needed to standard electrolyzer hardware.
This makes the solution practical and scalable for industrial use.
Major Cost and Energy Savings
The Princeton team ran detailed estimates comparing standard water-purification costs with their wastewater-acidification approach. They found:
- About 47% reduction in water-treatment cost
- About 62% reduction in water-treatment energy use
These savings are substantial, especially for large-scale hydrogen projects.
Large-Scale and Industry Implications
The researchers are now working with industry partners to study performance at larger scales and in real-world wastewater conditions. They are also testing whether this method could work with pretreated seawater, which could open another path to sustainable hydrogen in coastal regions.
Previously, the same group published analyses identifying optimal locations across the United States where hydrogen facilities could be co-located with wastewater treatment plants. These colocation models reduce not only water demand but also costs associated with piping, storage, and regional hydrogen distribution.
Why This Breakthrough Matters
This research stands out because it offers a practical, low-cost modification that can be applied directly to existing electrolyzer technology. It does not rely on exotic materials, specialized membranes, or complex reactor redesigns.
Its potential impact includes:
- Reduced strain on freshwater resources
- Lower cost of green hydrogen production
- New opportunities for sustainable industrial clusters
- More distributed hydrogen infrastructure
- Fewer environmental trade-offs in green hydrogen development
Given how central hydrogen is becoming for steel-making, fertilizer production, energy storage, and heavy transportation, improving water efficiency is a critical step toward real-world adoption.
Additional Context: Understanding Electrolysis and Water Quality
Since the topic relies heavily on electrochemical behavior, here’s foundational context that helps clarify why this research matters.
How PEM Electrolyzers Work
A PEM electrolyzer splits water using three main components:
- Anode – where water molecules give up electrons
- Cathode – where hydrogen is formed
- Membrane – a selective barrier allowing only protons (H⁺ ions) to flow through
The membrane’s ability to transport protons efficiently determines the electrolyzer’s stability and hydrogen output.
Why Membrane Fouling Happens
When water contains multivalent ions like Ca²⁺ and Mg²⁺, they tend to accumulate in the membrane’s channels. Over time, this:
- Blocks proton flow
- Increases resistance
- Raises operating temperature
- Lowers hydrogen production
- Causes irreversible damage
Ultrapure water avoids all of this—but at high cost.
Why Acidification Helps
An acidic environment is rich in free protons, which:
- Move quickly through the membrane
- Compete effectively with other ions
- Prevent scaling or deposition
- Keep the membrane clean and functional
This simple chemistry lies at the heart of the Princeton breakthrough.
Where the Research Is Heading Next
The team is exploring:
- Larger-scale pilots
- Long-term durability studies
- Integration with renewable energy systems
- Application to seawater-derived feedstocks
- How wastewater variations (seasonal or regional) affect performance
The researchers aim to support both scientific advancement and real-world industry needs.
Research Reference
Electrolytic Hydrogen Production from Acidified Wastewater Effluent
https://doi.org/10.1016/j.watres.2025.124672