Zirconia-Enhanced Ceramic Electrolytes Could Push Solid-State Batteries Toward Safer and Longer-Lasting Performance

Solid-state batteries have been sitting at the edge of a major leap for years, and now a fresh breakthrough from a partnership of four universities and three U.S. national laboratories suggests that leap may be getting closer. Led by The University of Texas at Austin, the research team has developed a new method to strengthen and improve garnet-based ceramic electrolytes, one of the most promising materials for next-generation all-solid-state lithium batteries. Their findings present significant improvements in performance, durability, and even manufacturing efficiency.
Why Solid-State Batteries Matter Right Now
Traditional lithium-ion batteries rely on organic liquid electrolytes, those sticky, maple-syrup-like solutions that allow lithium ions to flow between the electrodes. These liquids are efficient but come with a major downside: they’re flammable. They also represent the hydrocarbon “fuel” that sustains battery fires and thermal runaway events—the kind that make headlines when electronics or electric vehicles overheat.
Solid-state batteries replace these flammable liquids with solid ceramic electrolytes, which practically eliminates the biggest fire risk. Not only do solid-state systems promise improved safety, but they also unlock higher energy density, especially when paired with lithium-metal anodes. This combination is attractive for electric vehicles, drones, portable electronics, and other technologies where long life, fast charging, and safety are essential.
Still, ceramic electrolytes aren’t perfect. They can be expensive to manufacture, sensitive to quality-control issues, and vulnerable to lithium dendrites—microscopic metal filaments that can grow inside the battery, pierce the electrolyte, and cause short circuits.
The Issue With Garnet Electrolytes
Garnet-structured ceramics have been front-runners in the search for an ideal solid-state electrolyte. Their unique crystal framework allows lithium ions to move quickly and efficiently, which is exactly what you want for high-performance batteries. But even garnet electrolytes face recurring problems:
- Cracking, which tends to start at weak grain boundaries
- Void formation during sintering
- Dendrite penetration, especially along those flawed grain boundaries
- High processing temperatures, which raise manufacturing costs
The heart of the issue is mechanical fragility. Tiny cracks or defects inside the electrolyte become starting points for dendrites and electrical failure.
What This New Research Did Differently
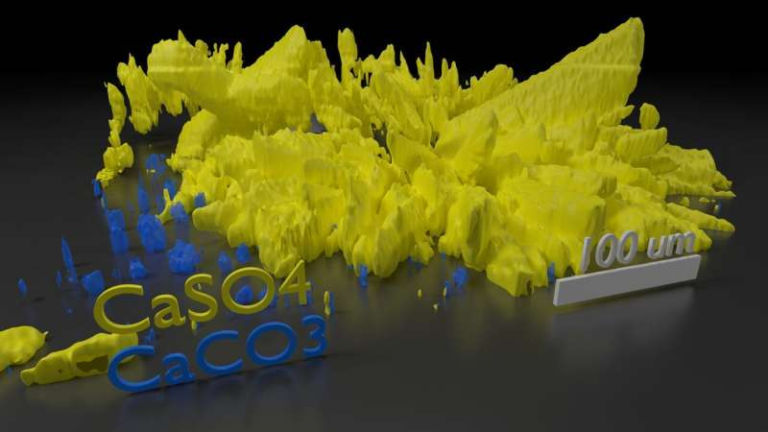
The team approached the problem the way a jeweler might refine a gemstone—by reinforcing weak points. Their solution centers on dispersing micro-scale zirconia (ZrO₂) particles throughout the garnet grains to strengthen the entire structure.
Zirconia is doing two jobs at once in this design:
- It improves densification during fabrication, closing gaps and reducing porosity.
- It stops or slows lithium dendrites from forming and advancing through the material.
What makes this possible is a clever method involving carbide additives. These additives decompose exothermically during synthesis, meaning they release heat from inside the material. This provides an internal thermal boost, allowing the garnet to form properly at lower external temperatures, which can significantly reduce production costs.
Why Zirconia Helps
The zirconia particles don’t just take up space—they reinforce the regions where problems typically begin. Grain boundaries are normally the most vulnerable locations in ceramics. When dendrites form, they often follow these boundaries, finding the path of least resistance. By embedding zirconia in these regions:
- Cracks have a harder time forming.
- Dendrites encounter a tougher, less reactive barrier.
- The electrolyte becomes more uniform and more mechanically robust.
Stronger Performance Backed by Testing
In laboratory tests, the zirconia-modified garnet outperformed the unmodified version in a significant way: it nearly doubled the critical current density, which is the maximum current the electrolyte can tolerate before short-circuiting occurs. This is a major milestone because solid-state batteries often struggle to support high charging currents without risking failure.
A higher critical current density means a battery can:
- Charge faster
- Deliver higher power
- Operate more safely under demanding conditions
And it can do so without sacrificing the core promise of solid-state technology: enhanced safety.
Why This Matters Beyond Batteries
While the primary focus is improving solid-state batteries, the researchers note that this advancement could benefit other sectors that rely on high-quality ceramics, especially where defect control is crucial. Industries involving sensors, fuel cells, aerospace components, and advanced electronics could all potentially leverage this approach.
The Role of Garnet Structure in Ion Transport
The base material here—garnet-type oxides like LLZO (lithium lanthanum zirconium oxide)—is already known for impressive ionic conductivity. The garnet structure includes pathways that allow lithium ions to hop from one site to another with minimal resistance. Enhancing this material with zirconia doesn’t disrupt these pathways; it reinforces them.
In other words, the ionic superhighway stays open, but now the “roadbed” is tougher.
What Are Lithium Dendrites and Why Are They a Problem?
Lithium dendrites are thin metal filaments that grow within a battery when lithium ions deposit unevenly during charging. In a liquid electrolyte battery, they move through the fluid and eventually bridge the two electrodes. In solid electrolytes, they exploit cracks, voids, or weak spots.
If dendrites reach the opposing electrode:
- The battery short circuits
- Heat can build rapidly
- Fire or failure may occur
The fact that zirconia reinforcement reduces dendrite growth is a big win for safety and device longevity.
A Quick Breakdown of the Materials at Play
Garnet Electrolyte (LLZTO):
- High ionic conductivity
- Stable against lithium metal
- Robust chemical properties
- Historically susceptible to cracking and dendrites
Zirconia (ZrO₂):
- High mechanical toughness
- Low electronic conductivity
- Excellent thermal stability
- Ideal for blocking dendrite paths
Carbide Additives:
- Used to trigger localized heat during sintering
- Lower external furnace temperature needs
- Support better densification
Challenges Still Ahead
Despite how promising this breakthrough is, the study authors make it clear that solid-state batteries still face barriers before widespread commercial use becomes a reality. Some ongoing challenges include:
- Creating robust interfaces between the electrolyte and electrodes
- Scaling up ceramic processing while keeping costs manageable
- Ensuring long-term cycling stability
- Preventing other forms of mechanical degradation over time
Even with a stronger electrolyte, building a full cell that performs consistently for years is its own engineering challenge.
Why This Research Is Exciting
The work offers a practical, materials-level solution that directly addresses some of the toughest issues in solid-state battery design. By improving safety, performance, and potential manufacturing efficiency all at once, the zirconia-modified garnet pushes the technology further toward real-world viability.
The combination of enhanced mechanical strength, dendrite resistance, and higher current handling makes this a meaningful step forward for the entire battery field.
Research Paper:
Grain boundary zirconia-modified garnet solid-state electrolyte (Nature Materials, 2025)